Antibodies show potential to boost fertility for women with ovarian failure
Mouse models show that an antibody therapy could normalise reproductive performance.
A Tsinghua University team led by Professor Bai Lu has recently shown that administering an antibody called Ab4B19 promotes the development of ovaries and enhances fertility in mouse models with a condition called premature ovarian failure.
Premature ovarian failure is a leading cause of infertility, affecting 1–5% of women aged under the age of 40, explains Lu. The disease occurs when the ovaries aren’t producing typical amounts of the hormone estrogen, or releasing eggs regularly. However, the mechanisms underlying the condition remain poorly understood, and there is no effective treatment.
Remarkably, treatment with the Ab4B19 antibody, which is also known to stimulate neuronal growth, completely reversed deficits in ovarian follicles, which are small sacs in the ovaries that release eggs and hormones. The treatment also normalized ovarian hormones and restored the number and quality of immature eggs in the mouse models1.
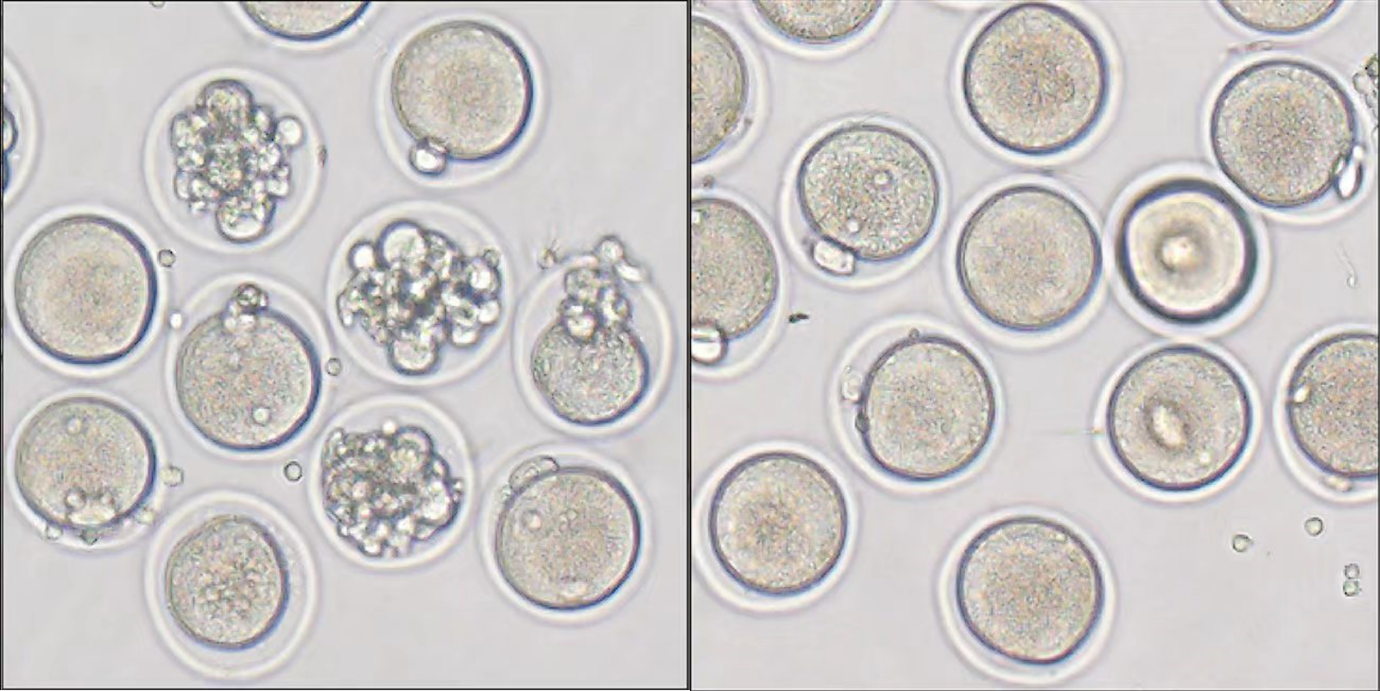
Here we see mouse cells, known as oocytes, that develop into eggs within mammalian ovaries. The black arrows point to abnormal oocytes from a cyclophosphamide-induced-premature ovarian failure model group (left), while oocytes from a cyclophosphamide-induced-premature ovarian failure group treated with Ab4B19 antibodies appear relatively normal. The scale bar is 150 μm.
In the study, Ab4B19 was shown to penetrate ovarian follicles via the bloodstream and activate a key receptor on brain-derived neurotrophic factor (BDNF) proteins, says Lu. The receptor, tropomyosin receptor kinase B (TrkB), regulates hormones during follicular development, among other things.
More targeted treatment
Lu’s keen interest in targeting BDNFs dates back to 2013, when he reviewed the possibilities for using BDNF-like molecules to treat neurodegenerative diseases2, which is his speciality area. BDNF is critical for neuronal growth and synaptic repair, explains Lu, a professor at the Tsinghua University School of Pharmaceutical Sciences. BDNFs have also been linked to premature ovarian failure in genetic association studies.
However, the protein itself cannot be used as a drug for several reasons. “One is that BDNFs are difficult to diffuse in tissues,” Lu says. Because of this, they remain at injection sites without penetrating deeply. Another reason is that the protein is easily degraded by enzymes in the body and so its half-life in the blood stream is less than two hours.
In addition, high local concentrations of BDNF may activate their other main receptor, called p75NTR, Lu explains. This receptor is prominently expressed in dying neurons linked to muscle movement (motoneurons), and its activation may have detrimental effects on ovarian follicles.
In 2019, Lu’s team published a paper outlining a strategy for using TrkB agonistic antibody Ab4B19 to activate BDNFs to treat motoneuron degeneration3. Using both cultures and mouse models, this study showed that administering the antibody didn’t activate p75NTR and it enhanced motoneuron survival.
In the premature ovarian failure study, Ab4B19 was also specifically activating TrkB, but not p75NTR, says Lu. And unlike BDNFs, the antibody exhibits a half-life of at least two weeks and diffuses readily in tissues.
Reversing damaging conditions
In the 2022 study, premature ovarian failure mouse models were injected with the antibody via their tail veins. After 16 days, this was shown to reverse the symptoms of the condition by normalising hormonal changes that injure the ovaries, preserving egg development and restoring the number and quality of immature eggs.
For example, in one of the mouse models, the antibody treatment corrected the disrupted cycle of ovulation. For both models, it reversed abnormalities in the level of the hormone estradiol. Both sets of mice showed improvements in shape and number of follicles. In addition, for one mouse model group, the proportion of females that delivered offspring was up 38.7% for the antibody-treated group.

Professor Bai Lu is from the Tsinghua University School of Pharmaceutical Sciences.
Wider fertility potential?
The TrkB receptor had never been considered as a drug target for the treatment of premature ovarian failure before, says Lu. But single-cell transcriptome analysis suggests that Ab4B19 may elicit similar effects to the mouse models in human cells.
Lu’s group has also confirmed that the antibody activates TrkB signalling in human cell cultures of human ovary tissue. The next step for the team is clinical trials to look at the response and for any side effects in humans who are experiencing premature ovarian failure.
The researchers also hope their treatment could one day help boost fertility in women. The group noted in their study that BDNF expression is down-regulated in follicles of older women.
“Given the data so far, we believe that an Ab4B19 treatment could contribute to improving fertility in premature ovarian failure patients, as well as increasing the reproductive capacity of pets, endangered species, and agricultural animals,” Lu says.
References
1. Qin, X., Zhao, Y., Zhang, T., Yin., C., Qiao, J. et al. TrkB agonist antibody ameliorates fertility deficits in aged and cyclophosphamide-induced premature ovarian failure model mice. Nature Communications 13, 914 (2022) doi: 10.1038/s41467-022-28611-2
2. Lu, B., Nagappan, G., Guan, X., Nathan, P., J., & Wren, P. BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases Nature Reviews Neuroscience 14, pages 401–416 (2013) doi: 10.1038/nrn3505
3. Guo, W., Pang, K., Chen, Y., Wang, S., Li, H et al. TrkB agonistic antibodies superior to BDNF: Utility in treating motoneuron degeneration Neurobiology of Disease 132, 104590 (2019) doi: 10.1016/j.nbd.2019.104590.
Editor: Guo Lili