Starving malaria parasites by blocking their sugar uptake could be the key to combating the looming threat of drug resistant strains.
In June 2021, for the first time the World Health Organization (WHO) certified China as malaria-free after it reported zero indigenous cases since 2017. In the same summer, Tsinghua researchers detailed a new anti-malarial avenue, a small molecule that blocks sugar uptake in the malaria parasite.
If China and other parts of the world wish to keep malaria at bay there is an urgent need for new treatments to battle emerging drug resistant strains, says Hang Hubert Yin, deputy dean of the School of Pharmaceutical Sciences at Tsinghua.
While artemisinin-based therapies, which emerged in the 1990s, have almost halved malaria’s enormous death toll since the 2000s, resistance to first-line defence artemisinin-based combination therapies have recently emerged in both South America and parts of Southeast Asia, just south of China. So Yin’s team have continued to work hard toward a solution.
Like most animal cells, malaria cells use sugar as their main source of energy, explains Yin. “Unlike previous methods to perturb the membranes or target the parasite’s DNA, this new approach attempts to selectively limit the parasite’s sugar intake.”
The key to the new strategy is to block the parasite’s sugar uptake without compromising the same physiological process in the host, explains Yin. The team hopes to do this by exploiting structural differences between the proteins mediating the transport of sugar across cell membranes in humans and Plasmodium falciparum, the deadliest of the four species of malaria.
P. falciparum has already developed resistance to most first-line antimalarial drugs currently in use.
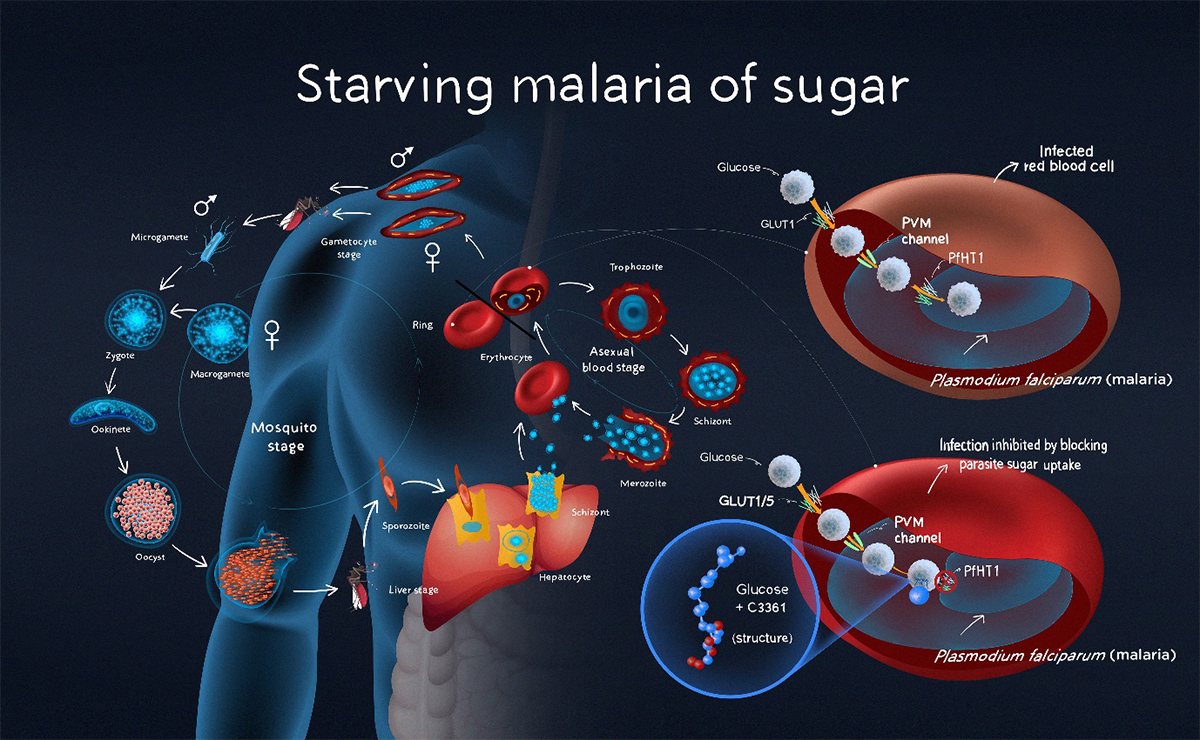
A Tsinghua team has designed a small molecule that inhibits the Plasmodium falciparum hexose transporter 1, the main sugar-uptake protein of the deadliest species of malaria, without depriving host cells of sugar. The possibility that this could slow malaria’s replication process in human blood is an important new avenue for development given the rise of drug resistant strains of the deadly parasite across the world, says lead researcher Hang Hubert Yin.
Structural sweet spot
In 2020, Yin and his colleagues resolved the atomic structure of PfHT1(Plasmodium falciparum hexose transporter 1), the parasite’s main sugar-uptake protein.
They found that when PfHT1 was bound to a moderately selective inhibitor, C3361, its structure re-arranged affecting the protein’s function. This discovery suggested that it could be possible to design more potent and selective agents that target both the protein’s orthosteric site, where the sugar substrate binds, and a newly identified site induced by the C3361 binding, also known as an allosteric site.
In their latest 2021 paper published in Proceedings of the National Academy of Sciences (PNAS), Yin’s team, in collaboration with researchers at Princeton University in the United States and the Global Health Discovery Institute (GHDDI) in Beijing, described the effects of a series of rationally designed, novel small molecules that bind to both the orthosteric and allosteric sites of PfHT1 simultaneously.
These compounds selectively inhibit PfHT1 more than the human glucose transporter 1 (hGLUT1) and effectively limit the growth of multiple strains of P. falciparum in human red blood cells.
“To the best of our knowledge, this is the very first successful case for simultaneously targeting both the allosteric and the orthosteric sites of a transporter,” says Yin. “Our findings demonstrate that PfHT1 is a druggable target and establish the basis for the rational design of next-generation antimalarial drugs.”

Hang Hubert Yin’s team is developing next-generation antimalarial strategies that exploit the structural differences between proteins that mediating sugar transport across cell membranes in humans and malaria.
China’s gatekeepers
China’s malaria free certification requires it to demonstrate the capacity to prevent the re-establishment of transmission.
Effective prevention and monitoring efforts are already reducing the risk of importing cases from neighbouring countries in which the disease is endemic, but work like Yin’s is necessary to ensure the looming threat of drug resistant strains is kept at bay too.
Yin says that there’s no room for complacency and his team looks forward to continuing to work with world-leading institutions such as GHDDI and Princeton University to validate their compounds’ clinical efficacy, and to develop new drugs against other infectious diseases using structure-based drug design.

Hang Hubert Yin is a deputy dean of the School of Pharmaceutical Sciences at Tsinghua University. Yin arrived at Tsinghua after receiving a Ph.D from Yale University, working as a post-doctoral researcher at the University of Pennsylvania and gaining tenure at the University of Colorado Boulder. He has received numerous awards including, among others, the American Chemical Society’s David W. Robertson Award for Excellence in Medicinal Chemistry and
Reference
Huang, J. et al. Orthosteric–allosteric dual inhibitors of PfHT1 as selective antimalarial agents. PNAS 118 (3), e2017749118 (2021) DOI: 10.1073/pnas.2017749118