Liu Xiangyu’s group in the School of Pharmaceutical Sciences, Tsinghua University, in collaboration with researchers from Stanford University, Friedrich-Alexander-Universität Erlangen-Nürnberg , and the University of California San Diego, recently reported on the crystal structures of the human β1AR bound to an antagonist carazolol and different agonists including norepinephrine, epinephrine and BI-167107, elucidatinghow the extracellular vestibule serves as a 'selectivity filter' to determine ligand subtype selectivity.
G protein-coupled receptors (GPCRs) are the largest family of membrane receptors with more than 800 family members in the human genome. GPCRs play important roles in physiology and they are the targets of approximately 30% of clinical drugs.The beta-adrenergic receptors (βARs) belong to the GPCR family.As part of the sympathetic nervous system, βARs mediate physiological responses to the catecholamines norepinephrine and epinephrine to regulate cardiovascular, respiratory and metabolic functions.
Norepinephrine is a neurotransmitter released from sympathetic nerves, while epinephrine is a hormone primarily released from the adrenal medulla into the systemic circulation. While epinephrine binds β1AR and β2AR with similar affinities, the smaller norepinephrine is approximately tenfold-times as selective for the β1AR. Under physiological conditions, β2AR activationshould be minimized to avoid opposing β1AR-mediated cardiac regulation. Thus, the lower affinity of the β2AR for norepinephrine appears to play an important role in cardiac physiology. This difference is surprising given that norepinephrine and epinephrine are very similar, except that epinephrine contains an additional methyl group, and the amino acids that form the orthosteric binding pocket are identical for β1AR and β2AR. The molecular mechanism of norepinephrine’sselectivity for β1AR over β2ARhas been a puzzle for decades.
To understand the molecular mechanism for this physiologically important phenomenon, the research group measured the binding kinetics of norepinephrine to both human β1AR and β2AR, as well as two chimeric constructs that switch the extracellular vestibules of β1AR and β2AR (named β1ARin/ β2ARoutand β2ARin/ β1ARout). The results suggest that the association rate rather than the dissociation rate determines the selectivity of norepinephrine to the receptors,andthe extracellular vestibules of the β1AR and β2AR are the key determinants of these different association rates (Figure1).
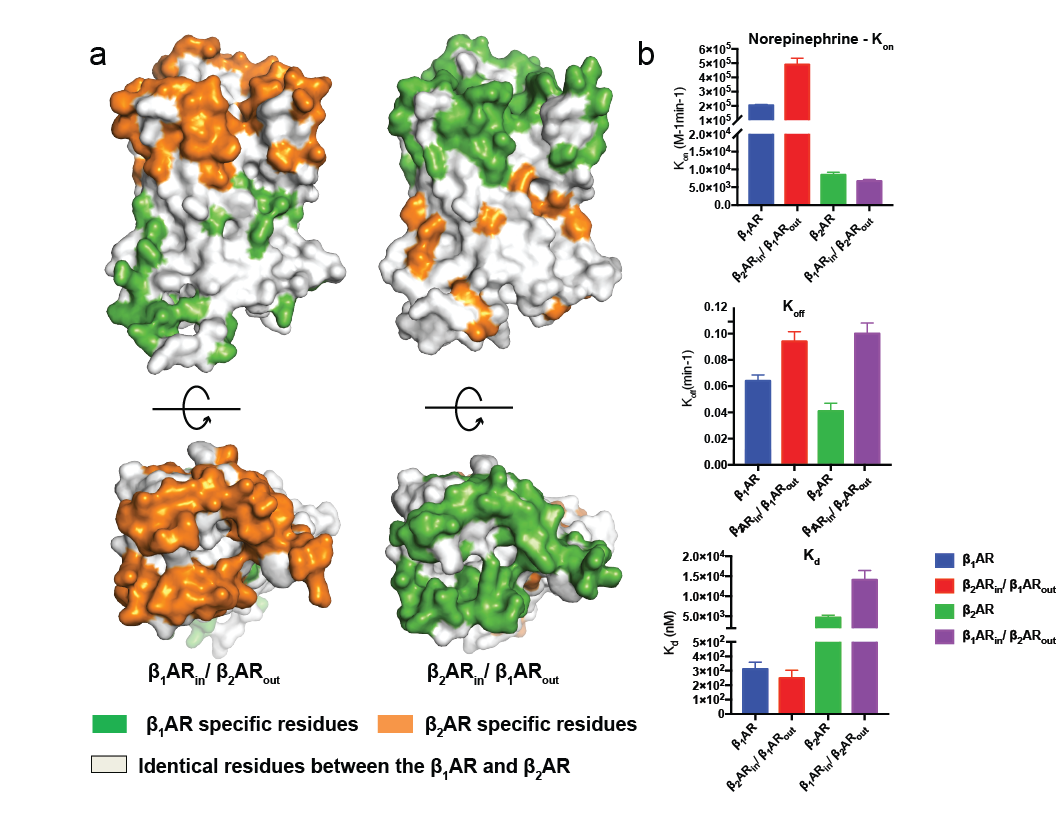
Figure 1. Kinetic studies of norepinephrine binding to the β1AR, β2AR and β1ARin/ β2ARout, β2ARin/ β1ARoutchimeras.
Delineating structural information on the extracellular vestibules is the key to understanding the molecular mechanism of norepinephrine selectivity. The structures of the human β1AR in both inactive and active conformations were determined, including the structures of the human β1AR bound to two endogenous ligands norepinephrine and epinephrine. Structural comparison revealed that the catecholamine-binding pockets are identical between β1AR and β2AR, but the extracellular vestibules have different shapes and electrostatic properties. Metadynamic simulations indicated that β1AR and β2AR have different ligand entrance pathways that account for the association rate difference. Interrogation of the entrance pathway through site-directed mutagenesis suggests that residues within the extracellular vestibule may serve as “selectivity filter” for norepinephrine. Metadynamic simulations revealed that epinephrine also takes different paths to bind to human β1AR and β2AR. However, the additional methyl group at its nitrogen alters the electron distribution on the catecholamine. Consequently, epinephrine does not show a preference for the extracellular vestibule of the β1AR or β2AR (Figure 2).
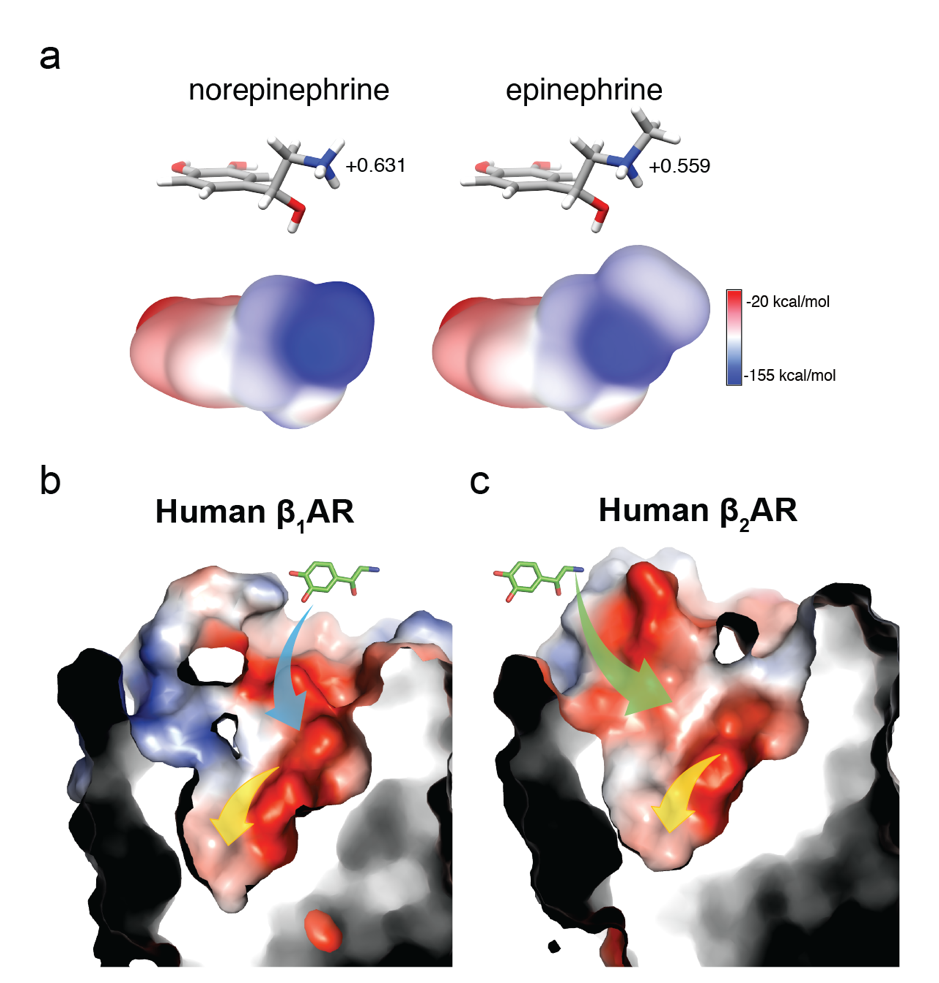
Figure 2. The ligand entrance pathways of the β1AR and the β2AR have different electrostatic properties.
This work addresses an interesting and physiologically important difference in the binding affinity of norepinephrine for the human β1AR and β2AR.The results also have a broader implication for drug development where receptors with identical orthosteric pockets could have different selectivity filters that define the pharmacology. Identifying subtype-selective drugs is a major goal in the development of safer and more efficacious therapeutics that target GPCRs. This work suggests that efforts to develop subtype-selective ligands using structure-based drug design should consider the extracellular vestibule not only in the context of allosteric modulators or bitopic ligands, which bridge both the orthosteric site and the vestibule, but also for smaller orthosteric ligands.
The workrecently published in Cell Research, entitled “Binding pathway determines norepinephrine selectivity for the human β1AR over β2AR”. Itis the result of a collaboration between Professor Xiangyu Liu’s group at Tsinghua University, Professor Brian Kobilka’s group at Stanford University, Professor Peter Gmeiner’s group at Friedrich-Alexander-Universität Erlangen-Nürnberg and Professor Roger Sunahara’s group at University of California San Diego. Professor Xiangyu Liu, Professor Peter Gmetiner and Professor Roger Sunahara are corresponding authors of the paper. Dr. Xinyu Xu from School of Life Science, Tsinghua University, Dr. Jonas Kaindl from School of Medicinal Chemistry, Friedrich-Alexander-Universität University and Dr. Mary J. Clark from School of Medicine, University of California San Diego are co-first authors of the paper. The work is supported by grants from Beijing Advanced Innovation Center for Structural Biology and Tsinghua-Peking Joint Center for Life Sciences. Crystal diffraction and data collection was supported by Spring-8 synchrotron radiation facility, Japan.
Source: School of Pharmaceutical Sciences
Editors: Lin Lu, John Olbrich

