Hongwei Wang Group Reported the CryoEM Structure of Yeast Cytoplasmic Exosome Complex on Cell Research
In July 2016, Cell Research published a research article titled “CryoEM Structure of Yeast Cytoplasmic Exosome Complex” by Professor Hongwei Wang at School of Life Sciences and his colleagues. Prof. Wang has led a group to study macromolecular complexes in the eukaryotic RNA quality control system for years and they have previously reported the multiple conformations and degradation pathways of yeast RNA Exosome complex on PNAS and Nature Structural and Molecular Biology.
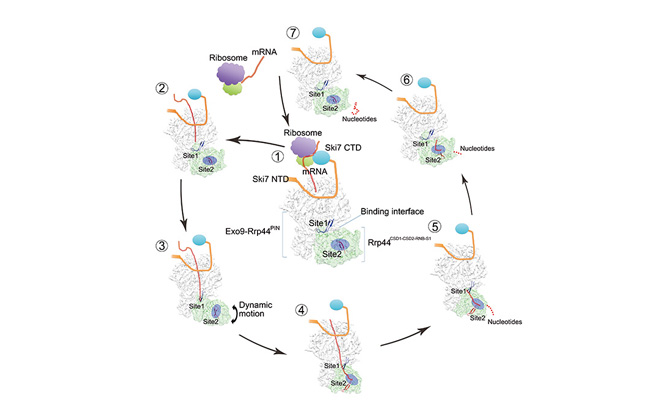
The Working Mechanism of Cytoplasmic Exosome-Ski7 complex.
RNAs in eukaryotes play important roles in various processes such as translation, gene regulation and RNA interference. However, during the synthesis or processing of RNAs, some RNA molecules with defect may accumulate. The RNA exosome can recognize the aberrant RNAs and degrade them promptly with the assistance of specific cofactors. RNA exosome is also responsible for the turnover of mRNAs and other RNAs. The yeast RNA exosome consists of a highly conserved 9-subunit core complex and two catalytic subunits, Rrp44 and Rrp6. Rrp44 is bound with exosome core both in cytoplasm and nucleus, while Rrp6 is unique in nucleus. The yeast cytoplasmic exosome has a Ski7 protein that bridges the complex to Ski complex for degradation of aberrant mRNAs.
In the current work, Hongwei Wang and his colleagues solved the cryo-electron microscopy structure of yeast Exosome-Ski7 complex at two distinct functional states at near-atomic resolution, providing new insights to the mechanism of cytoplasmic RNA decay in eukaryotes. The structures of Exosome-Ski7 with or without RNA substrates revealed the key motif involved in the conformational switch of the complex induced by RNA substrates. The structure in combination with additional biochemical assays also illustrates the exclusive nature of Ski7 and Rrp6 proteins interacting with Exosome complex, explaining the distinct RNA decay mechanism in dependence of cellular localization of RNA substrates.
Hongwei Wang is the corresponding author of this paper. Graduate students Junjie Liu (School of Life Sciences & PTN, 2011), Chuya Niu (School of Life Sciences, 2012) and Postdoctoral Fellow Yao Wu (NIBS) are co-first authors of this work. Two undergraduate students, Yang Wang and Mingda Ye, at School of Life Sciences also participated in part of this work. The research groups of Mengqiu Dong and Niu Huang in NIBS, Quansheng Liu in IHEP (CAS), Xuerui Yang and Junbiao Dai in THU all contributed in this work. The China National Center for Protein Sciences Beijing and “Explorer 100” cluster system of Tsinghua National Laboratory for Information Science and Technology provided facility support. This work was supported by the National Natural Science Foundation of China and the National Basic Research Program of China. Hongwei Wang is also a Principal Investigator of the Tsinghua-Peking Joint Center of Life Sciences.
Original paper:http://www.nature.com/cr/journal/vaop/ncurrent/full/cr201656a.html
Related papers:http://www.pnas.org/content/104/43/16844.short
http://www.nature.com/nsmb/journal/v21/n1/full/nsmb.2736.html

