Dr. Wanli Liu’s Research Group from Tsinghua Published Article in Nature Communications, Revealing New Mechanism of IgG-B Cell Receptor Activation Regulated by Acidic phospholipids
The research group led by Dr. Wanli Liu from School of Life Sciences, Tsinghua University and, their collaborators, Dr. Chenqi Xu’s group from the Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences have reported the reversible dynamic interaction between acidic phospholipids and mIgG cytoplasmic tail (mIgG-Tail), providing a novel mechanism to govern the burst-enhanced activation of IgG-B cell receptor (IgG-BCR). These findings have been published in Nature Communications online on October 8th, 2015 under the manuscript entitled "Acidic phospholipids govern the enhanced activation of IgG-B cell receptor”.
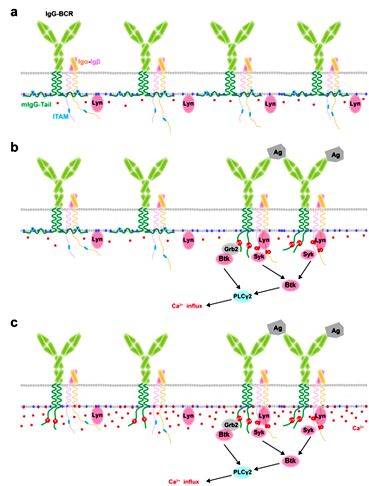
Dynamic interaction between mIgG-Tail and acidic phospholipids governs the enhanced activation of memory B cells
The pathogens, encountered by the body, render the specialized memory cells to “remember” them. Such memory immunity is a hallmark of the adaptive immunity and also as the basis for almost all current vaccines. Memory B cells that express the isotype-switched IgG-BCR are in charge of the establishment of the rapid and high-titered memory IgG antibody response. Numerous studies have highlighted how IgG-BCR arms itself into a highly effective signal transduction machine. An essential but unanswered question is how does the IgG-BCR prevent illegitimate signaling?
In this report, Dr. Liu’s group systematically addressed this question and showed that the positively-charged mIgG-Tail specifically interacts with negatively-charged acidic phospholipids in the inner leaflet of the plasma membrane (PM) via ionic protein-lipid interaction and thus the key signaling tyrosine in ITT motif of the mIgG-Tail was sequestered in the membrane hydrophobic core, which help quench mIgG-Tail basal level signaling in quiescent IgG+ memory B cells. They also found that the membrane-sequestered mIgG-Tail can be released by antigen engagement or Ca2+ mobilization in the initiation of IgG+ B cell activation, which then enhances Ca2+ mobilization, and in turn facilitates more release of mIgG-Tail to further enhance memory B cell activation through such a positive feedback mechanism.
This study shed new light on a novel regulatory mechanism of IgG+ memory B cell to balance the immunoprotection and immunopathology and provide new perspective of self-tolerance in class-switched IgG+ memory B cells, which will help in further understanding the memory immunity and in the design of new vaccines for infectious diseases and drugs against autoimmune diseases and B cell tumors.
Xiangjun Chen from School of Life Sciences, Tsinghua University and Weiling Pan from the Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences are the co-first authors. Dr. Wanli Liu and Dr. Chenqi Xu are the co-corresponding authors.
Dr. Wanli Liu joined School of Life Sciences, Tsinghua University after his Postdoctoral training in NIH in December, 2011. Dr. Wanli Liu’s group combines immunological and biochemical experimental approaches with cutting-edge high-resolution high-speed live cell and molecule imaging techniques to investigate the function of lymphocyte cells and related immune diseases. This paper is the new contribution to the filed of trans-membrane signal transduction of B lymphocytes after the publications of his lab in Science Signaling (2012), Journal of Immunology (2014) and Journal of Leukocyte biology (2015).
This research was funded by NSFC, Chinese Ministry of Science and Technology, Thousand Youth Talents Plan, Tsinghua-Peking Center for Life Science and the collaborative innovation center for diagnosis and treatment of infectious diseases.
The manuscript can be accessed by the following link:
http://www.nature.com/ncomms/2015/151006/ncomms9552/full/ncomms9552.html

