Dr. Bing Zhou's Research Group Published Article in PNAS, Revealing the Molecular Mechanism of Copper Ion Mediating Huntington Disease Progression
Dr. Bing Zhou’s group in School of Life Sciences, Tsinghua University, published a research article entitled “Huntington disease arises from a combinatory toxicity of polyglutamine and copper binding” in Proceedings of the National Academy of the Sciences of the United States of America (PNAS) (early publication online) on August 26th, 2013, reporting the molecular mechanism of copper ion mediating Huntington disease (HD) progression. The Ph.D. student Guiran Xiao from School of Life Sciences, Tsinghua University is the first author of this work and Dr. Bing Zhou is the corresponding author.
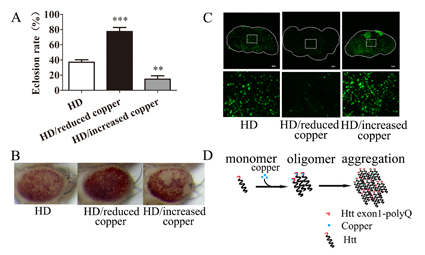
Figure 1 Modulation of copper transporters alters a spectrum of phenotypes associated with HD flies.
Huntington disease, which is used to be called Huntington’s chorea, is a neurodegenerative disorder caused by expansion of polyglutamine (polyQ) repeats within the functionally enigmatic huntingtin (Htt) protein. The etiology of this disease is not well clarified and effective therapeutics for HD is lacking. So it is very important to discover the pathogenic mechanism of this disease and search the new target for drug treatment and therapy.
Here using a Drosophila model of HD, wherein Htt exon1 with expanded polyQ (Htt exon1-polyQ) is introduced, they show that altered expression of genes involved in copper metabolism significantly modulates the HD progression. Intervention of dietary copper levels also modifies HD phenotypes in the fly. Copper reduction to a large extent decreases the level of oligomerized and aggregated Htt. Strikingly, substitution of two potential copper-binding residues of Htt, Met8 and His82, completely dissociates the copper-intensifying toxicity of Htt exon1-polyQ. Their results therefore indicate HD entails two levels of toxicity: the copper-facilitated protein aggregation as conferred by a direct copper binding in the exon1 and the copper-independent polyQ toxicity. The existence of these two parallel pathways converging into Htt toxicity also suggests that an ideal HD therapy would be a multipronged approach that takes both these actions into consideration.
This work was supported by the National Basic Research Program of China and the National Natural Science Foundation of China.

