The research group led by Professor Nieng Yan in School of Medicine, Tsinghua University, reported the crystal structure and functional mechanism of a Major Facilitator Superfamily membrane transporter in Nature by Advanced Online Publication (AOP) on September 26th, 2010. The hardcopy was published two weeks later.
Membrane proteins play an essential role in life. They mediate the exchange of nutrients, metabolites, energy, and signals across the hydrophobic lipid bilayer. Major Facilitator Superfamily (MFS) proteins represent an ancient and widespread family of secondary active transporters that are conserved from bacteria to human being. Deficiency of MFS transporters is associated with human diseases such as diabetes and seizures. Therefore, information on the structure and mechanisms of MFS transporters is essential both for elucidating membrane biology and for understanding diseases. Decades of investigations on MFS transporters led to the structural determination of merely three MFS transporters, those of LacY, GlpT and EmrD, with the first two in an inward-open conformation and the last one in an intermediate conformation. The lack of an MFS transporter in the outward-open conformation has largely restricted the mechanistic understanding of MFS transporters.
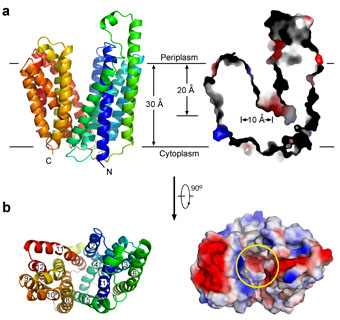
In their study, Professor Yan and colleagues reported the long-sought-after structure of an MFS transporter, the E.coli fucose:proton symporter FucP, in the outward-open conformation at 3.1 ?resolution. There are several unanticipated novel findings, and structural analysis suggests a transport mechanism for FucP ?alternating access by a concentric, rigid-body rotation of the N and C domains in the opposite orientations. Their structure fills an important void in understanding the transport mechanisms of MFS proteins.

