Pluripotency refers to the potential of a cell to differentiate into all cell types in a body. During early mammalian development, embryos undergo the first cell fate decision during the blastocyst stage, forming the inner cell mass (ICM) and trophectoderm (TE). The ICM will generate all embryonic lineage and the entire body. TE will primarily give rise to placenta. Pluripotency is believed to initially emerge in the ICM and gradually transits through several states: naive, formative, and primed pluripotency, ultimately giving rise to lineage differentiation. Pioneer transcription factors, such as OCT4 and SOX2, play crucial roles in the regulation of pluripotency. They are believed to bind and actively open closed chromatin to initiate gene transcription. Yamanaka and others demonstrated that by introducing the transcription factors OCT4, SOX2, KLF4, and MYC, terminally differentiated somatic cells can be reprogrammed into induced pluripotent stem cells (iPSCs). However, how pioneer factors like OCT4 and SOX2 regulate pluripotency network was primarily studied using in vitro-cultured embryonic stem cells. The precise mechanisms by which these factors regulate pluripotency programs in vivo remain elusive to date.
On December 15, the research group led by Professor Wei Xie from the School of Life Sciences at Tsinghua University published a research article in the journal Science, titled "Multifaceted SOX2-chromatin interaction underpins pluripotency progression in early embryos." This study revealed the regulatory mechanisms through which the pioneer factor SOX2 drives pluripotency progression in early mammalian embryos. The researchers discovered that in early mouse embryos, SOX2 does not simply function through the pioneer factor model by opening closed chromatin to activate pluripotency program. Instead, SOX2 exhibits multifaceted binding modes with chromatin that includes “Settler,” “Pioneer,” and “Pilot” binding. An active role of SOX2 in establishing a pluripotency regulatory network begins to emerge in late blastocysts when SOX2 opens widespread naïve pluripotency enhancers through “Pioneer binding”. Prior to this stage, a “Pre-pluripotency” cell state exists in early blastocysts when SOX2 primarily promotes gene expression through “Settler binding”, as it binds pre-accessible enhancers that are opened by early-stage expressing TFs. Therefore, such multifaceted chromatin interaction modes allow SOX2 to connect early developmental program executed by upstream transcription factors (e.g., NR5A2 and TFAP2C) before it adopts a more proactive role in establishing the naïve pluripotency.
By optimizing CUT&RUN technology, the researchers first captured the chromatin binding of the pluripotency factor SOX2 in mouse embryos, from day 3.5 (E3.5) to day 7.5 (E7.5), covering the entire process of pluripotency program progression. Using Sox2 knockout embryos and embryonic stem cells, the researchers systematically investigated the role of SOX2 in gene expression and chromatin opening during the establishment and transition of pluripotency. Their results revealed that the interaction between SOX2 and chromatin during early development does not follow a simple pioneer factor model. Instead, SOX2 exhibits several distinct chromatin interaction modes, including "settler binding," "pioneer binding," and "pilot binding." In E3.5 ICM, SOX2 primarily binds to pre-opened enhancers ("settler binding") and promotes ICM-specific gene expression. These pre-accessible enhancers are partially opened by early-expressing transcription factors such as TFAP2C and NR5A2. NR5A2 was recently identified as a key TF regulator of early ICM and TE programs in totipotent embryos by the same group (Lai et al., Cell Research, 2023). These data suggest that E3.5 ICM is in a distinctive "pre-pluripotency" state between totipotency and pluripotency. During this period, the pluripotency regulatory network is primitive, and pioneer pluripotency factors have not yet taken a dominant role. However, through binding to pre-open chromatin, SOX2 facilitates a successful transition from upstream developmental programs to pluripotency program. Subsequently, E3.5 cells enter the naive pluripotency of the epiblast at day 4.5 (E4.5) and the formative pluripotency of the epiblast at day 5.5 (E5.5). At this point, SOX2 undergoes genomewide relocation and massively opens chromatin to initiate the naive and formative pluripotency programs ("pioneer binding"). Additionally, SOX2 can also bind to a subset of enhancers without fully opening them. Instead, it prepares these enhancers for rapid opening for the next developmental stage ("pilot binding").
In summary, this study reveals how key transcription factors drives pluripotency programs in vivo and how pioneer factor exert their impacts through “settler-pioneer-pilot” chromatin interactions rather than a simple “pioneer” binding mode. A distinctive “pre-pluripotency” state was also identified in early blastocysts when the pluripotency network is still primitive and pluripotency key factors have not established dominant roles and their inter-dependency to maintain the pluripotency network. This data will lay the foundation for a better understanding of molecular control underlying pluripotency and cell fate determination, and the nature of in vivo and in vitro pluripotency models.
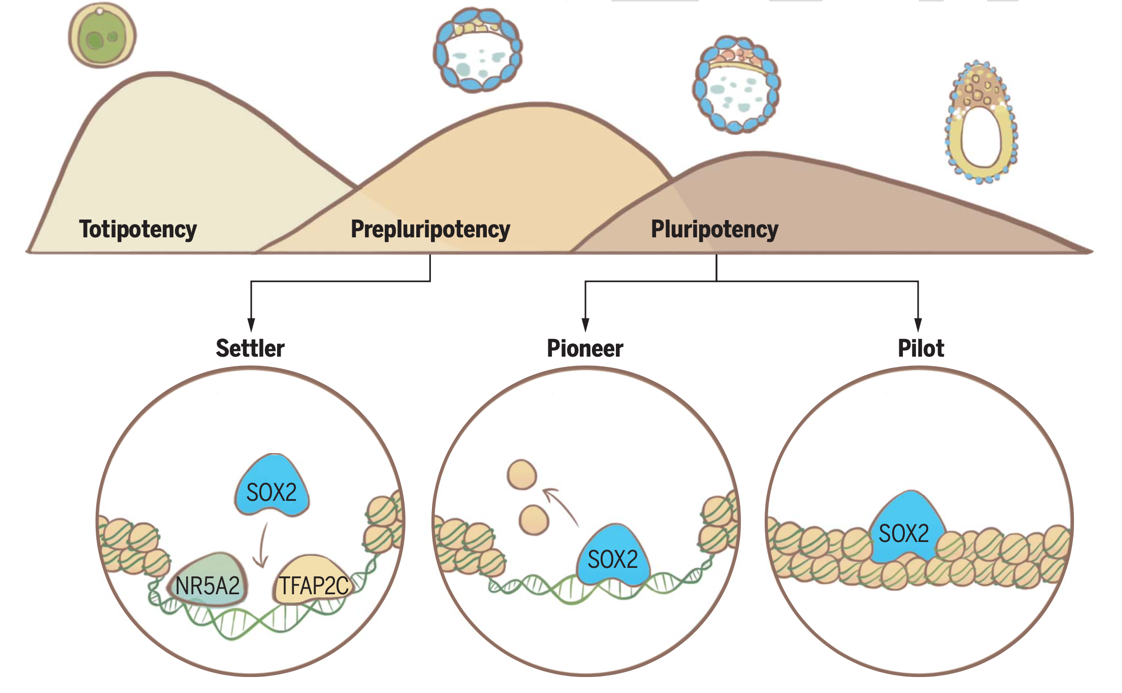
SOX2 regulatory circuitry during pluripotency progression reveals multifaceted master TF-enhancer interaction.
Prof. Wei Xie is the corresponding author of this paper. Ph.D students Lijia Li, Fangnong Lai, Xiaoyu Hu, and a postdoctoral fellow Bofeng Liu from the School of Life Sciences at Tsinghua University are co-first authors. This study also received support from the Animal Center and the Bioinformatics core facility at Tsinghua University. Dr. Bofeng Liu is a ShuiMu Scholar in Tsinghua University. Funding for this research was provided by the National Natural Science Foundation of China, the Key Research and Development Program of the Ministry of Science and Technology, and the Tsinghua-Beijing Life Science Center. Prof. Wei Xie is an HHMI International Research Scholar and a New Cornerstone Investigator.
Editor:Li Han

