Recently, Ligong Chen's group of the School of Pharmaceutical Sciences, Tsinghua University and their collaborators jointly discovered that the hepatic mitochondrial carrier protein SLC25A47 activates AMPKα-mediated lipid metabolism homeostasis, and its expression defect is involved in the occurrence and development of non-alcoholic fatty liver disease (NAFLD) and hepatocellular carcinoma (HCC).
NAFLD is currently recognized as the most common chronic liver disease worldwide, which has brought a heavy burden to the world public health. SLC transporters are widely expressed in various tissues of the human body, with numerous members widely distributed on cell membranes and various organelle membranes, playing a "metabolic gatekeeper" role in various tissues. Genome-wide association analysis has found that SLC transporters play a crucial role in human diseases, and their expression is tissue-specific and may become a new target for the treatment of metabolic diseases.
SLC25A47 regulates lipid metabolism homeostasis through activating AMPKα
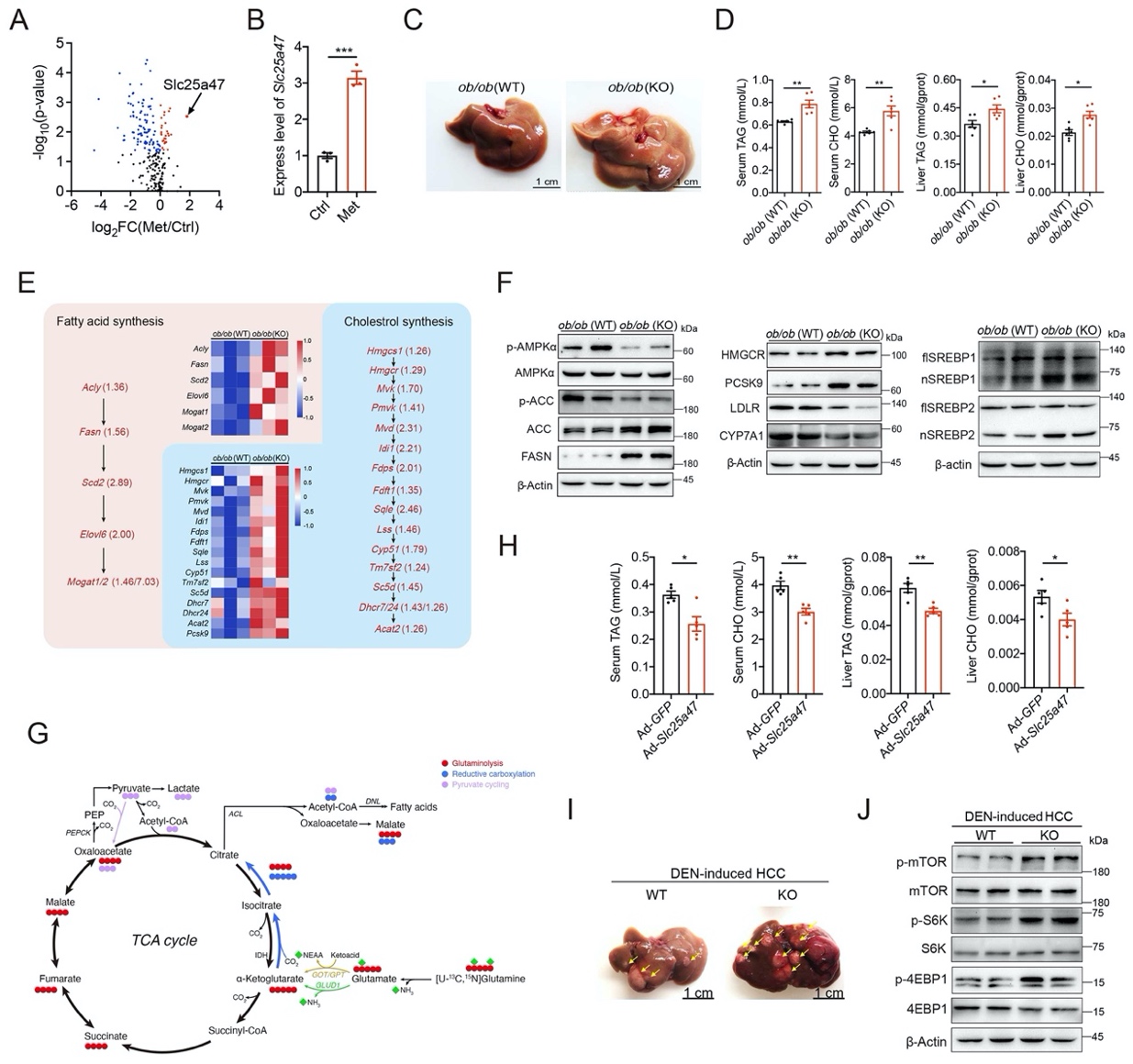
Metformin can improve NAFLD in preclinical animal models by increasing the activity of the cellular energy sensor AMPKα. The study found that metformin affects the expression of multiple SLC transporters, among which the change in SLC25A47 is the most significant (Figure 1A, B). SLC25A47 is specifically and highly expressed in liver tissue, but its transport substrates and role in physiological metabolic regulation remain to be further studied.
The study used CRISPR/Cas9 technology to construct Slc25a47 gene knockout (KO) mice and found that Slc25a47-KO mice showed more obvious liver lipid accumulation under the conditions of methionine-choline-deficient feed or high-fat feed. Subsequently, the research team constructed ob/ob (Slc25a47-KO) mice and found that compared with the control group ob/ob (WT) mice, ob/ob (Slc25a47-KO) mice had increased lipid accumulation in the liver (Figure 1C), increased triglyceride (TAG) and cholesterol (CHO) levels in serum and liver tissue (Figure 1D). RNA-sequencing results showed that compared with ob/ob (WT) mice, the expression of multiple genes related to fatty acid and cholesterol synthesis increased in the liver of ob/ob (Slc25a47-KO) mice (Figure 1E). Finally, it was found that Slc25a47 knockout promotes the activation of the sterol regulatory element binding proteins (SREBPs) by inhibiting AMPKα phosphorylation, thereby regulating the expression of many lipogenic genes, promoting lipid synthesis, and causing more lipid accumulation in the liver of ob/ob (Slc25a47-KO) mice (Figure 1F). Furthermore, the research team used 13C-metabolic flux analysis to find that the primary hepatocytes of Slc25a47-KO mice mainly use amino acids to provide energy for lipid synthesis (Figure 1G). In addition, overexpression of Slc25a47 in mouse liver can inhibit SREBPs activation by promoting AMPKα phosphorylation, slowing down lipid accumulation in the liver of high-fat diet mice (Figure 1H).
Since the fatty acid synthase (FASN) was defined as a tumor antigen for aggressive breast cancer in the 1990s, more and more studies have shown that the biosynthesis of fatty acids is crucial for the growth and survival of cancer cells. Therefore, this study speculated that the involvement of SLC25A47 in HCC may also be related to lipid metabolism. Previous research has shown that the chemical carcinogen DEN can induce HCC in mice, and its pathogenesis is similar to human HCC. By constructing a DEN-induced HCC mouse model, this study found that Slc25a47 knockout promoted liver fat accumulation in mice, which in turn promoted the pathogenesis of HCC (Fig. 1I, J).
Homology model and substrate validation of SLC25A47
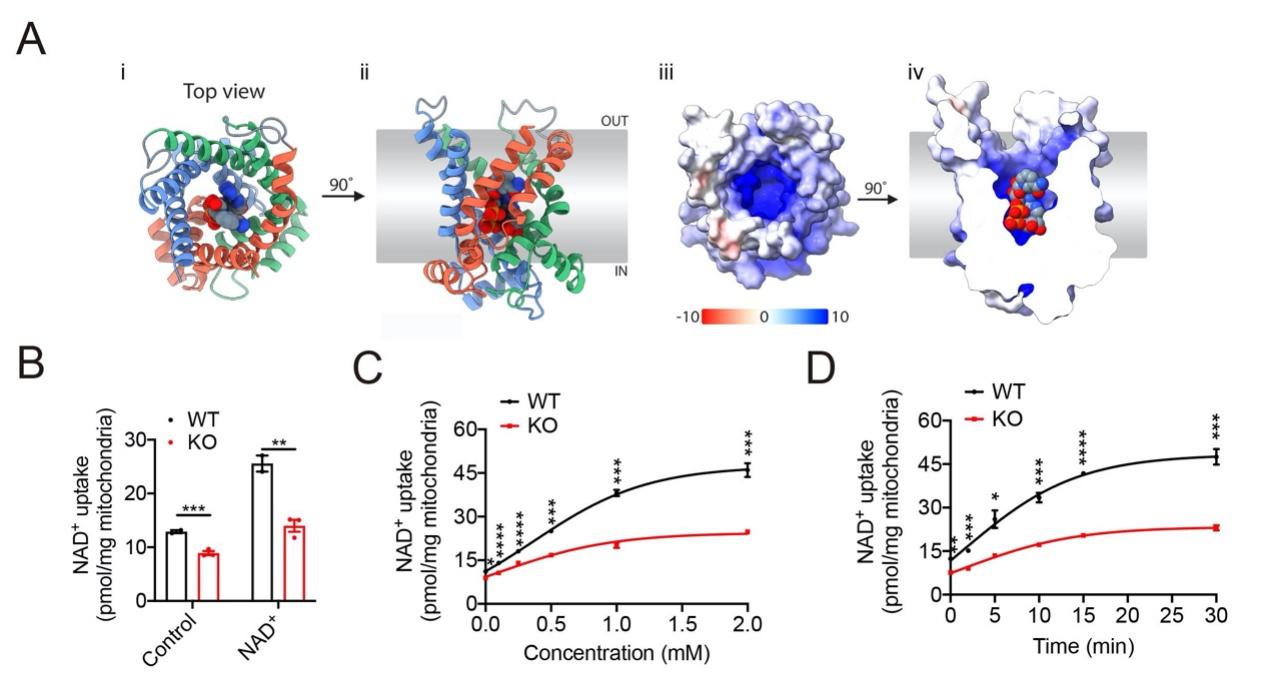
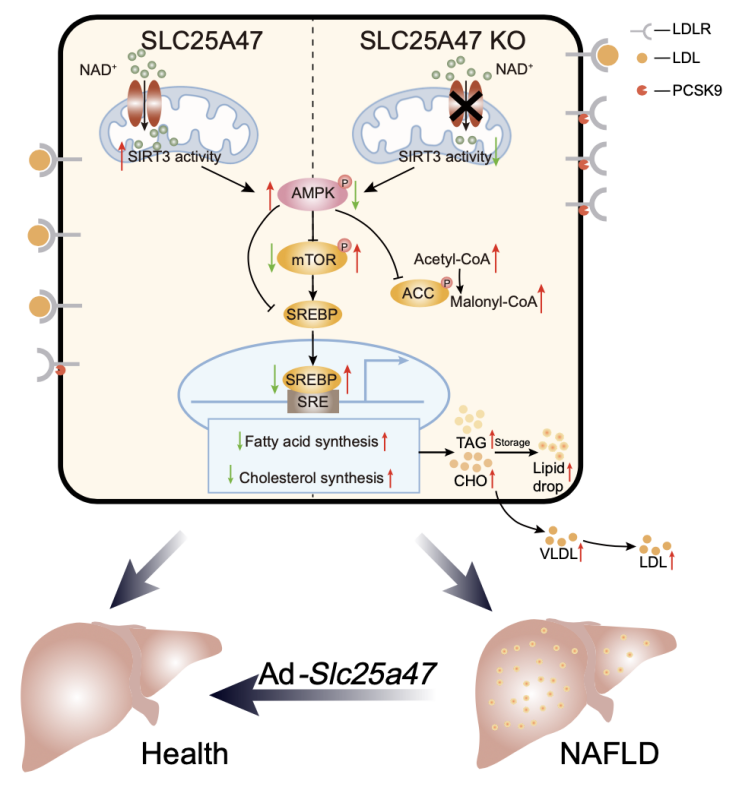
In addition, based on the results of the metabolomics testing in this study, and the virtual screening of the human metabolic database using a homology model of human SLC25A47 predicted and optimized by AlphaFold2, a series of candidate substrates were obtained, including NAD+, adenine, FAD, taurine, as well as various acylcarnitines, CoA, amino acids, etc. NAD+ was experimentally identified as one of the transport substrates of SLC25A47. SLC25A47 regulates the activity of SIRT3 by regulating the level of NAD+ to mediate the activation of AMPKα (figure 2). These findings indicate that SLC25A47 is a hepatic mitochondrial NAD+ transporter, which regulates lipid metabolism through the SIRT3-AMPKα-SREBPs pathway (Figure 3). In summary, this study provides a potential drug target for the clinical treatment of NAFLD and HCC, and provides further experimental evidence for the development of drugs or gene therapies targeting SLC25A47 transporter.
A proposed model for the role of SLC25A47 in regulating hepatic lipid metabolism
The research was recently published online in the Hepatology with the title "Hepatic mitochondrial NAD+ transporter SLC25A47 activates AMPKα mediating lipid metabolism and tumorigenesis".
Associate Professor Ligong Chen of the School of Pharmaceutical Sciences and Professor Hao Fan of the Singapore A*STAR are co-corresponding authors of this paper. Dr. Lili Cheng of the School of Pharmaceutical Sciences is the first author of the article. Dr. R.N.V. Krishna Deepak of the Singapore A*STAR, Dr. Guoqiang Wang, Ziyi Meng, Lei Tao and others from the School of Pharmaceutical Sciences are co-authors. This research was supported by the National Natural Science Foundation of China, Ministry of Science and Technology of China National Key R&D Programs, Tsinghua-Foshan Innovation Special Fund, and Tsinghua Precision Medicine Fund.
Link to the paper: https://doi.org/10.1097/hep.0000000000000314
Editor: Li Han

