Mammalian oocyte maturation is a special biological process in which gene transcription is arrested, and mainly regulated by gene translation. At present, most of the omics studies on oocyte maturation are based on the transcriptional expression level, which cannot accurately reflect the translational expression level of genes. The profiling of translatome requires a large number of cells as materials or needs to express exogenous epitope tags, which cannot be used in the study of human oocytes.
To comprehensively reveal the transcription-translational dual-omics profile during human oocyte maturation, Kehkooi Kee's research group from the School of Medicine at Tsinghua University, Xiaoyan Liang's research group from the Reproductive Center of the Sixth Affiliated Hospital of Sun Yat-sen University, and a number of domestic reproductive centers cooperated to develop a transcription-translation dual-omics that is suitable for a single oocyte. The dual-omics technology (T&T-seq) can simultaneously detect total mRNA and translated mRNA in a single oocyte sample (Fig. 1), and further study the translational regulatory mechanisms during human oocyte maturation. The technique is also applicable to other cell types, including human embryonic kidney cells (293FT). In addition to revealing the transcription-translational dual-omics profile of human oocytes for the first time, this study identified the OOSP2 secreted protein to promote oocyte maturation in vitro, providing a new technical means for clinical assisted reproductive technology. The related research was titled "Single-cell transcriptome and translatome dual-omics reveals potential mechanisms of human oocyte maturation" published online in Nature Communications on August 30, 2022.
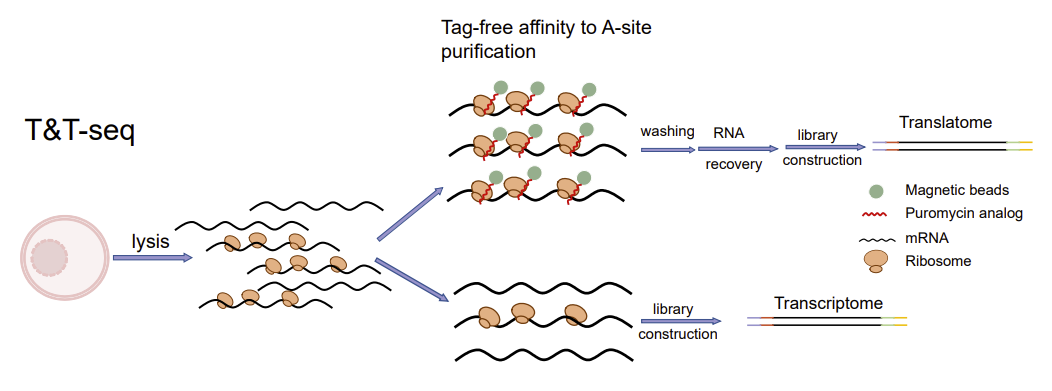
Fig. 1. Schematic diagram of T&T-seq
Highlights of the findings include:
1. Revealing the regulation of spatiotemporal order during human egg maturation
In immature oocytes, the functions of genes that are actively translated are mainly ribosome formation, mitochondrial synthesis, and cytoplasmic translation. In mature oocytes, the functions of genes that are actively translated are mainly chromosome segregation, cell cycle regulation, and chromosome modification (Fig. 2). These findings reveal the sequential regulation of gene expression from the cytoplasm to the nucleus during oocyte maturation. Moreover, the genes with high translation efficiency in mature oocytes were significantly more than those in immature oocytes, reflecting the importance of translation regulation during oocyte maturation.
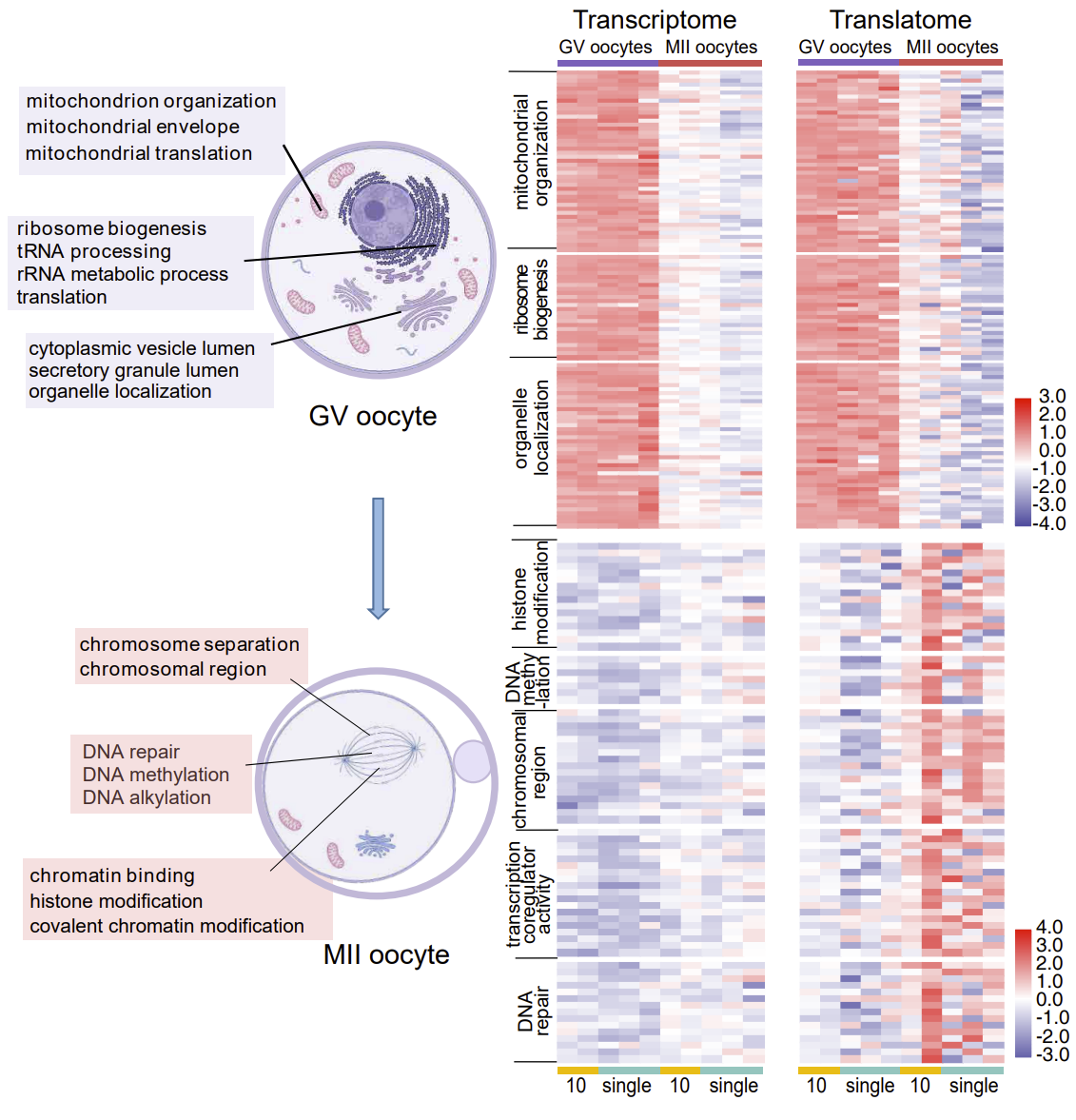
Fig. 2 The regulation of spatiotemporal order during oocyte maturation
2. Comparison of translational expression patterns during maturation of mouse and human oocytes
Mouse models are often used to study and infer the mechanisms of human oocyte maturation; however, there are some unique translational expression patterns that may only exist in human oocytes, so conclusions drawn from mouse data alone may not be applicable to human oocytes. Based on T&T-seq of mouse and human oocytes, the authors compared and contrasted the translation sets of these two species, and identified some genes with different translational patterns, such as those of the OOSP protein family. In the mouse OOSP family, both OOSP1 and OOSP3 proteins are up-regulated during maturation, while OOSP2 is down-regulated during maturation. In the human OOSP family, however, the opposite is true. The authors found that the mouse OOSP2 gene has only one PAS motif (RNA sequence element at 3’ untranslated region of mRNA), while both OOSP1 and OOSP3 have multiple CPE motifs (RNA sequence element at 3’ untranslated region of mRNA) adjacent to the PAS motif. In contrast, the human OOSP2 is the only OOSP gene that carries both CPE and PAS (Fig. 3).
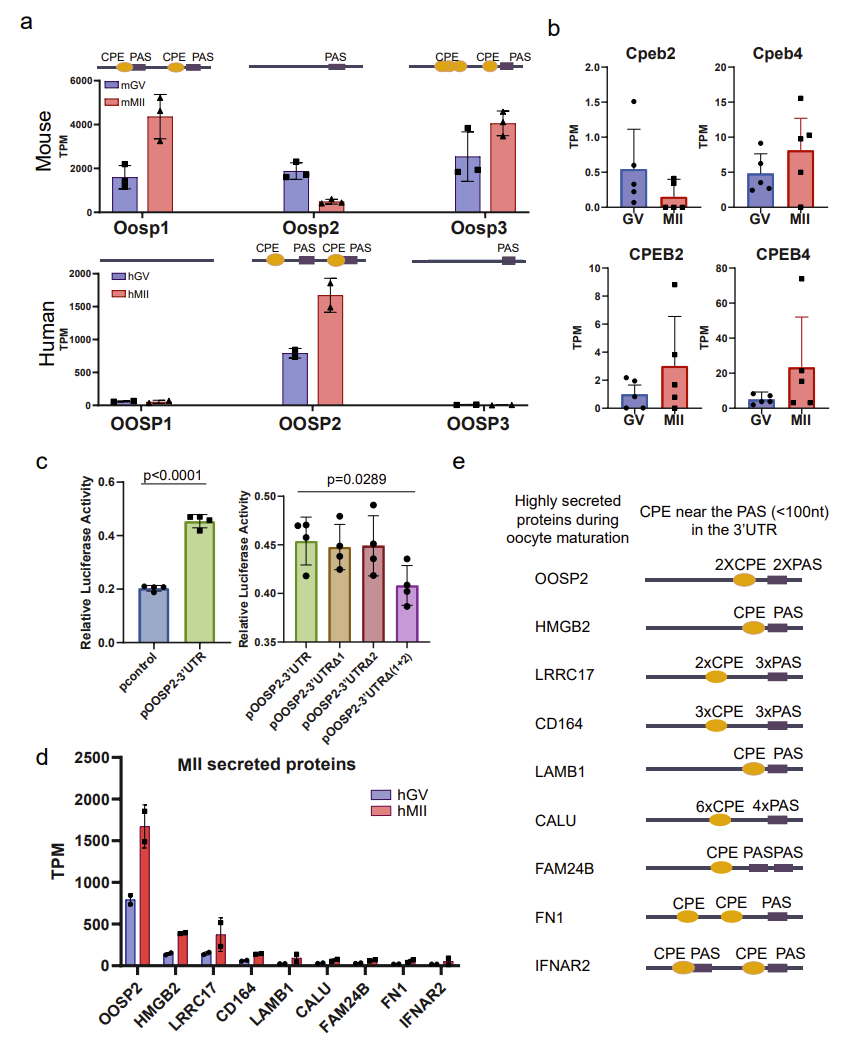
Figure 3. CPE sites and PAS motifs may regulate the translation of secreted proteins
3. Adding recombinant OOSP2 protein can improve the in vitro maturation rate of human oocytes
To explore whether OOSP2 has a regulatory role in oocyte maturation, the authors added recombinant OOSP2 proteins into GV oocytes collected from donors and monitored oocyte maturation using an incubator with time-lapse microscopy. Nuclear envelope rupture (GVBD) and polar body expulsion (PBE) are two stages of morphological changes after oocyte maturation under the microscope (Fig. 4). Oocytes are considered mature when PBE is observed. The authors compared paired GV oocytes from the same donor in each set of experiments to eliminate heterogeneity between different donors, and recorded the time to GVBD and the time to PBE of the experimental and control oocytes, respectively. The authors found that the addition of the OOSP2 protein increased the rate of in vitro maturation of human oocytes. In contrast, the addition of OOSP2 antibodies to the GV oocytes blocked the function of the OOSP2 protein, thereby inhibiting the maturation process of the oocytes.
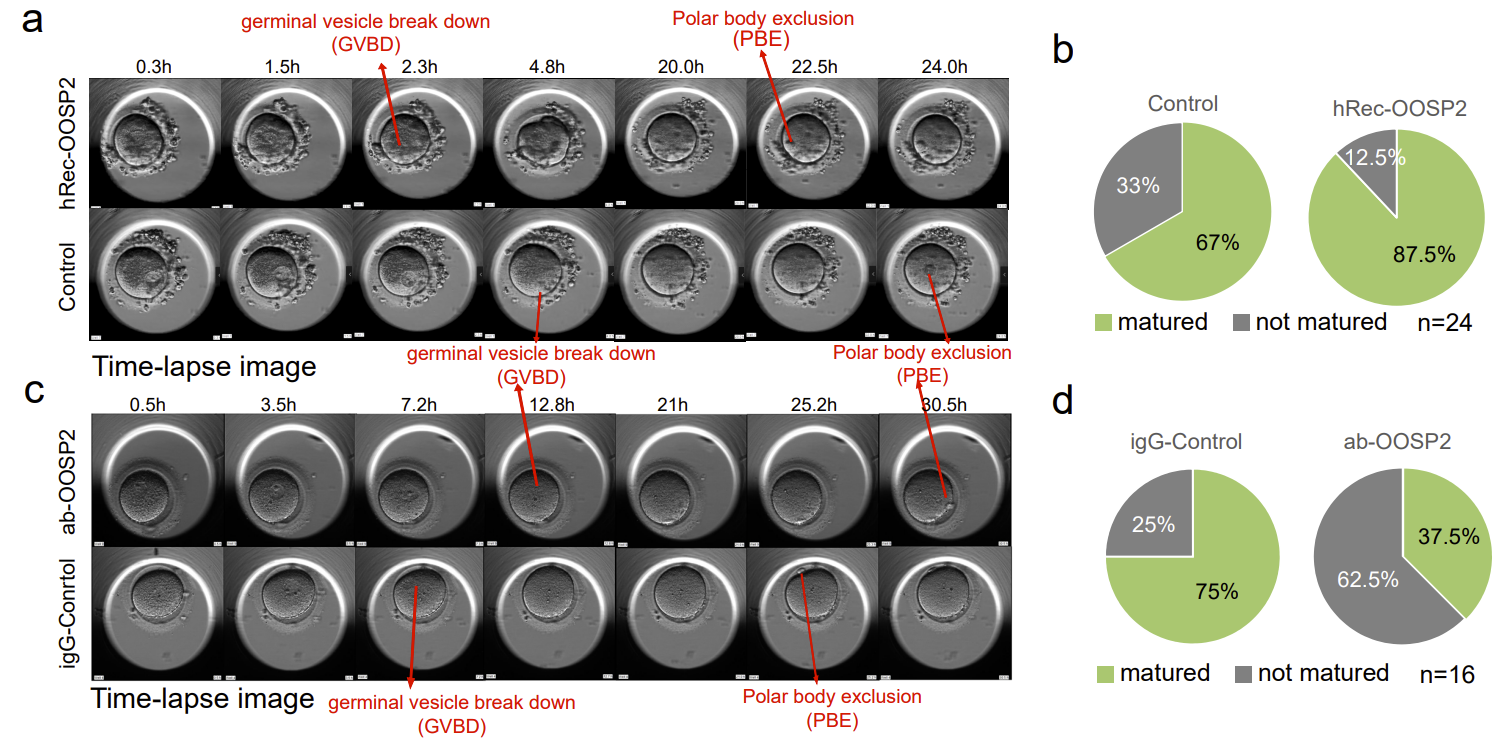
Fig. 4. OOSP2 protein promotes in vitro maturation of human oocytes
In summary, this study developed T&T-seq for a single oocyte, revealing for the first time a dual-omics profile and the spatiotemporal sequential regulation of human oocyte maturation; revealing the similarities and differences of the translatome profiles during oocyte maturation of mouse and human species; elucidating the regulatory role of CPE motifs on the translation of secreted proteins during oocyte maturation; identifying that OOSP2 secreted proteins play an important role in oocyte maturation; revealing the possibility of using OOSP2 to promote the maturation of oocytes by regulating the translation of proteins related to the small GTPase signaling pathway, and provide a new technical method and theoretical basis for improving the clinical application of oocyte in vitro maturation.
Hu Wenqi, a graduate student of the School of Medicine, Tsinghua University, and Zeng Haitao, chief physician of the Reproductive Center of the Sixth Affiliated Hospital of Sun Yat-sen University, are the co-first authors of the paper. Professor Kehkooi Kee from the School of Medicine, Tsinghua University, and Professor Xiaoyan Liang from the Reproductive Center of the Sixth Affiliated Hospital of Sun Yat-Sen University are the co-corresponding authors of the paper. Professor Richeng Chian's research group from the Shanghai Tenth People's Hospital of Tongji University, Dr Lingbo Cai's research group of the First Affiliated Hospital of Nanjing Medical University, Professor Keliang Wu's research group of the Cheeloo College of Medicine, Shandong University, and Associate Professor Wuhua Ni's research group of the First Affiliated Hospital of Wenzhou Medical University participated in the preliminary research work of the project. This project is supported by the key research and development project of the Ministry of Science and Technology (2017YFC1001600).
Original link: https://www.nature.com/articles/s41467-022-32791-2
Editor: Li Han

