Natural gas hydrates (NGH), also known as “combustible ice”, are formed naturally in the presence of methane (CH4) and water (H2O) within suitable high-pressure and low-temperature conditions. Widely dispersed in marine locations and in or below permafrost, they have been identified as a future clean energy resource with huge potential. Since 2019, a research group led by Prof. Daoyi Chen from the Tsinghua Shenzhen International Graduate School (SIGS) Institute for Ocean Engineering has been developing technologies that could couple NGH recovery with carbon dioxide (CO2) geological sequestration processes for the dual benefit of clean energy and a low-carbon future.
Based on the geological properties of hydrate reservoirs located in the South China Sea, possible marine conditions were identified where CH4-CO2-N2 mixed hydrates could potentially form. The group thus proposed a novel method to recover CH4 and sequestrate CO2 synergistically under high-pressure and high-temperature marine conditions. To test the above hypothesis, a series of experiments were designed and conducted to recover CH4 by a short-term depressurization process followed by the injection of CO2/CO2-N2 gas mixtures in a specially-designed high-pressure (>15.0 MPa) reactor. Time-dependent pressure and temperature data, gas chromatography results and in-situ hydrate morphology images were acquired for data analysis. The group also developed a numerical method by estimating the partition of all components (CH4, CO2, N2 and H2O) in various phases (gas, liquid and hydrate) to quantify the dynamic behavior of hydrate formation, dissociation and replacement processes.
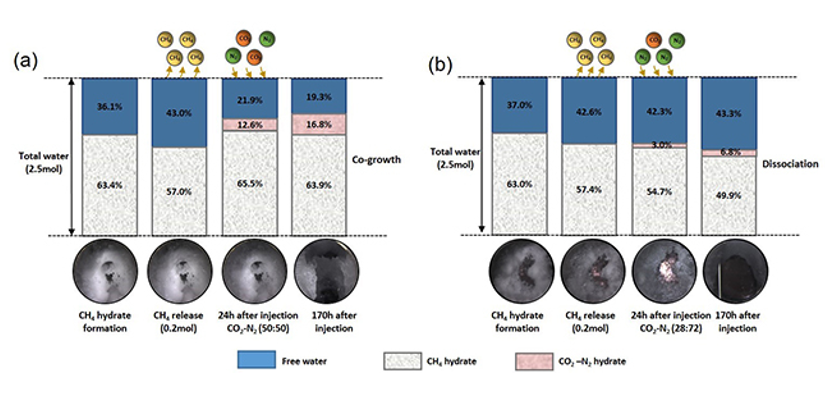
Fig. 1. Schematic of the evolution of H2O during the entire process and the corresponding hydrate morphology images in (a) co-growth mechanism and (b) dissociation mechanism
The study revealed both the thermodynamic and kinetic feasibility of CH4 recovery under marine conditions by combining depressurization with the injection of CO2/CO2-N2 gas. The presence of free CH4 in the system in a way assisted the sequestration of CO2/N2 as hydrate mixtures. In addition, direct injection of CO2/CO2-N2 would result in either the co-growth of mixed hydrate or the dissociation of CH4 hydrates largely depending on the gas composition.
This study was recently published in the Chemical Engineering Journal under the title “Effectiveness of CO2-N2 injection for synergistic CH4 recovery and CO2 sequestration at marine gas hydrates condition” with Tsinghua SIGS Ph.D. student Mengya Niu as the first author. Experimental results and findings from this study could provide guidance on the design of CH4 recovery and CO2 sequestration tests in future field production trials at marine conditions in South China Sea. The study was supported by the Guangdong MEPP Fund, Shenzhen Municipal Bureau of Planning and Natural Resources, and the Tsinghua SIGS International Joint Research Fund.
Link to full article: https://www.sciencedirect.com/science/article/pii/S138589472101202X
Writer: Mengya Niu
Editor: Karen Lee, Li Han

