Hydrogen energy is a pivotal clean energy carrier for achieving carbon neutrality. The Hydrogen Evolution Reaction (HER) via electrocatalytic water splitting is the fundamental process for green hydrogen production, where the development of high-efficiency and robust catalysts remains the central challenge. While noble metal-based catalysts exhibit superior activity, their high cost and scarcity limit large-scale industrial deployment. Recently, the research team led by Assistant Professor Deng Bing at the School of Environment, Tsinghua University, in collaboration with domestic and international partners, has utilized Flash Joule Heating (FJH) technology to develop a series of high-performance, stable catalysts with low noble metal loading, providing critical support for the advancement and application of green hydrogen technologies.
To address the long-term stability of HER catalysts, the team proposed an interfacial confinement strategy. Utilizing millisecond-scale FJH technology, the team conducted the efficient synthesis and activity tailoring of a “chainmail-like” catalyst, featuring isolated platinum atoms anchored on tungsten carbide nanocrystals encapsulated inside carbon nanotubes (Pt1/WCx@CNTs). This design not only boosts catalytic activity but also significantly enhances durability. The catalyst exhibits prominent catalytic performance toward acid HER with a low overpotential of 45.2 mV at 10 mA cm–2 and long-term durability over 500 h of continuous running. Mechanism studies reveal that the strong metal–support interaction on Pt1/WCx optimizes the charge redistribution at the Pt1–W2C interface and the hydrogen adsorption/desorption behavior. This study offers a potential avenue for the ultrafast and activity-controllable synthesis of highly stable single-atom catalysts. These findings were published as a cover article in Nano Letters on February 26, 2025, titled “Confined Flash Pt1/WCx inside Carbon Nanotubes for Efficient and Durable Electrocatalysis.”
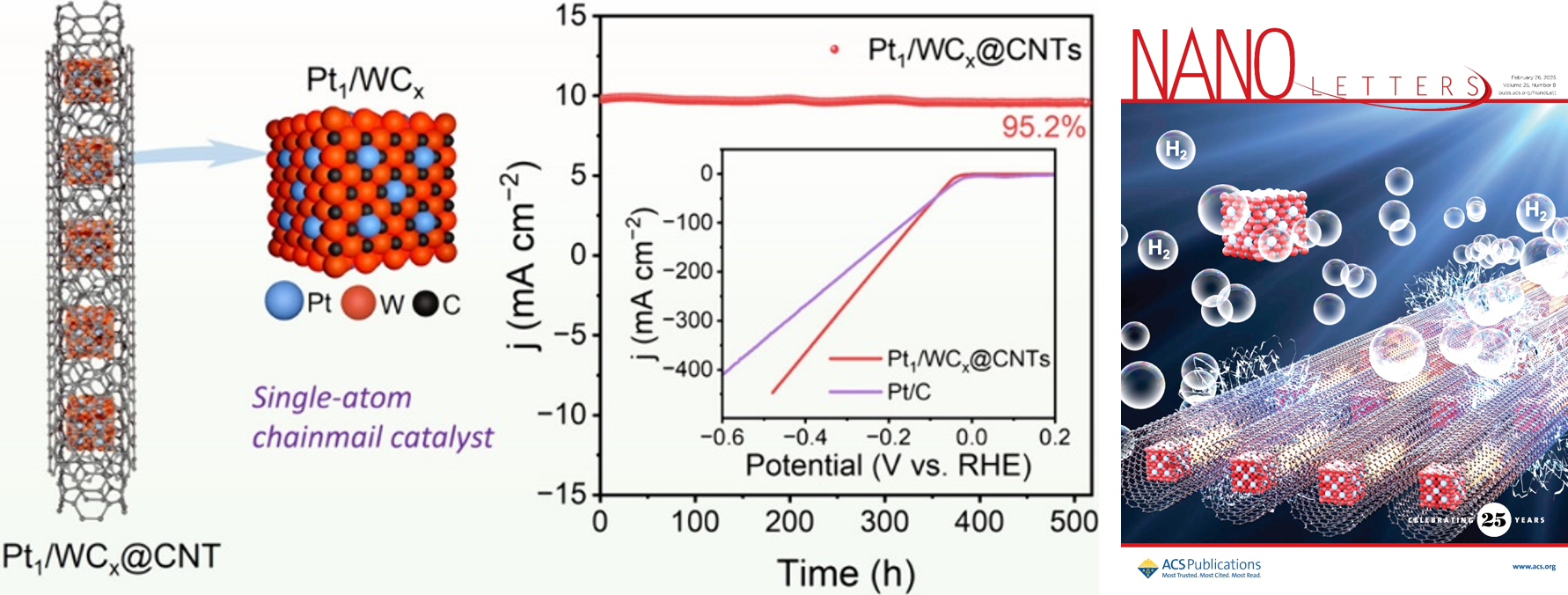
Fig 1. Confined Pt-WCx materials for high-efficiency and stable electrocatalysis.
Amorphous nanomaterials offer a high density of unsaturated active sites and unique electronic structures. However, their controllable synthesis often requires complex processes. The team developed an ultrafast quenching strategy using FJH to condense metal precursors into ternary platinum-nickel-phosphorus (PtNiP) amorphous nanoparticles (ANPs) within milliseconds. Through compositional optimization, the Gibbs free energy of hydrogen adsorption for Pt4Ni4P1 ANPs was tuned to 0.02 eV, a quasi-ideal value that surpasses even the benchmark metallic platinum catalyst. As a result, the catalyst exhibited superior activity in acidic electrolytes (η10 ∼ 14 mV, Tafel slope ∼ 18 mV dec–1), with a mass activity five times higher than state-of-the-art Pt/C. Life-cycle assessment and techno-economic analysis suggest that this approach enables notable reductions in greenhouse gas emission, energy consumption, and production cost for practical electrolyzer catalyst manufacturing. This work was published in the Journal of the American Chemical Society (JACS) on May 5, 2025, titled “Coupling Amorphization and Compositional Optimization of Ternary Metal Phosphides toward High-Performance Electrocatalytic Hydrogen Production.”
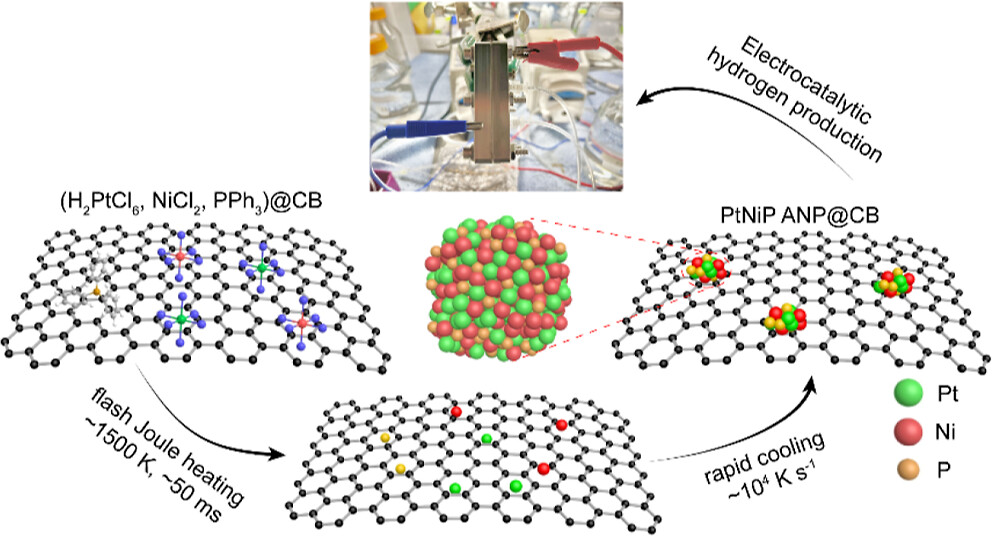
Fig 2. Coupling amorphization and compositional optimization for high-performance green hydrogen production.
The research team also achieved the precision synthesis of sub-nanometer molybdenum carbide (MoCx) wires through ultra-fast thermal processing. Utilizing single-walled carbon nanotubes (SWNTs) as both thermal conductors and structural templates, they synthesized one-dimensional MoCx nanowires via carbothermal reduction of encapsulated polyoxometalate cluster arrays—without the need for solvents, catalysts, or special gases. The resulting carbide-encapsulated SWNTs stabilizes iron phthalocyanine molecules, creating a catalyst that performs exceptionally in alkaline oxygen reduction reactions (ORR) with a half-wave potential of 0.91 V. Additionally, the team developed a single-site Pt-loaded MoCx chainmail catalyst, achieving a mass activity of 4.84A/mgPt and stability exceeding 350 hours in acidic HER. This research was published in ACS Nano on August 4, 2025, titled “Strong Interactions Between Flash Sub-Nanometer Carbide Nanowires and Single-Walled Carbon Nanotubes for Catalysis.”
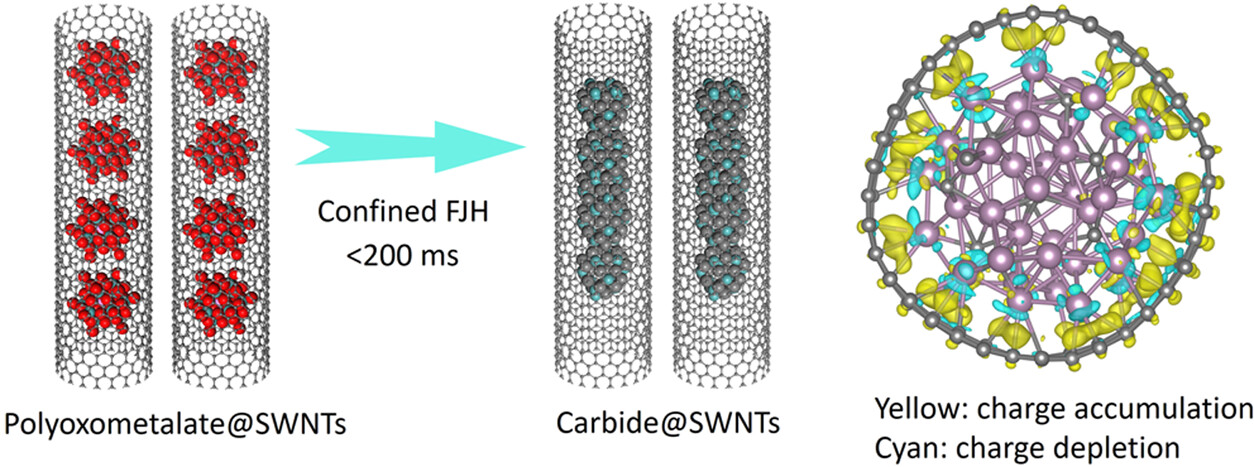
Fig 3. Strong interfacial interactions between flash carbide nanowires and SWNTs for high-efficiency catalysis.
Assistant Professor Deng Bing is the corresponding author for this research series. Key collaborators include researchers from Shanxi University, Southern University of Science and Technology, Rice University, and Corban University. The research was supported by the National Key R&D Program of China, the National Natural Science Foundation of China (NSFC), the Beijing Natural Science Foundation, and the Tsinghua University Initiative Scientific Research Program.
Paper Links:
https://doi.org/10.1021/acs.nanolett.4c05097
https://doi.org/10.1021/jacs.5c00071
https://doi.org/10.1021/acsnano.5c11080





