On January 9, 2023, the team of associate professor Miao Li of the School of Environment in Tsinghua University published a research paper entitled “N-doped carbon-iron heterointerfaces for boosted electrocatalytic active and selective ammonia production” online in the Proceedings of the National Academy of Sciences of the United States of America.
Nitrate (NO3-) ions, are one of the most widespread water pollutants in the world and poses a great threat to drinking water safety and human health. Therefore, from an environmental and energetic point of view, the electrochemical conversion of NO3- to ammonia (NH3) is a facile and efficient purification method and will provides a sustainable route to restore the balance of the global nitrogen cycle and plays a significant role in the environmental and economic impact of sustainable NH3 synthesis. The use of electrochemical methods to convert NO3- to NH3 not only reduces nitrate pollution, but also produces valuable ammonia. This provides a sustainable pathway to restore the global nitrogen cycle balance and plays an important role in the environmental and economic impact of sustainable ammonia synthesis. However, the development of electrode materials with the advantages of low cost, high activity and selectivity is a continuous challenge and pursuit in this field. In this study, the weak adsorption energy of the C active site on the reactant molecules weakened the reaction kinetics, thus reducing the removal efficiency and selectivity. Herein, we constructed a catalyst with new active sites by doping with nitrogen, which activated neighboring carbon atoms and enhanced metal-to-carbon electron transfer, resulting in high catalytic activity. We report a rational design of Fe nanoparticles wrapped in N-doped carbon (Fe@N10-C) as a high NH3 selective and efficient electrocatalyst using a metal-organic framework precursor. As a result, the Fe@N10-C nanoparticles with optimal doping of N demonstrated remarkable performance, with a record-high NO3- removal capacity of 125.8 ± 0.5 mg N gcat-1 h-1 and nearly 100 % (99.7 ± 0.1%) selectivity.
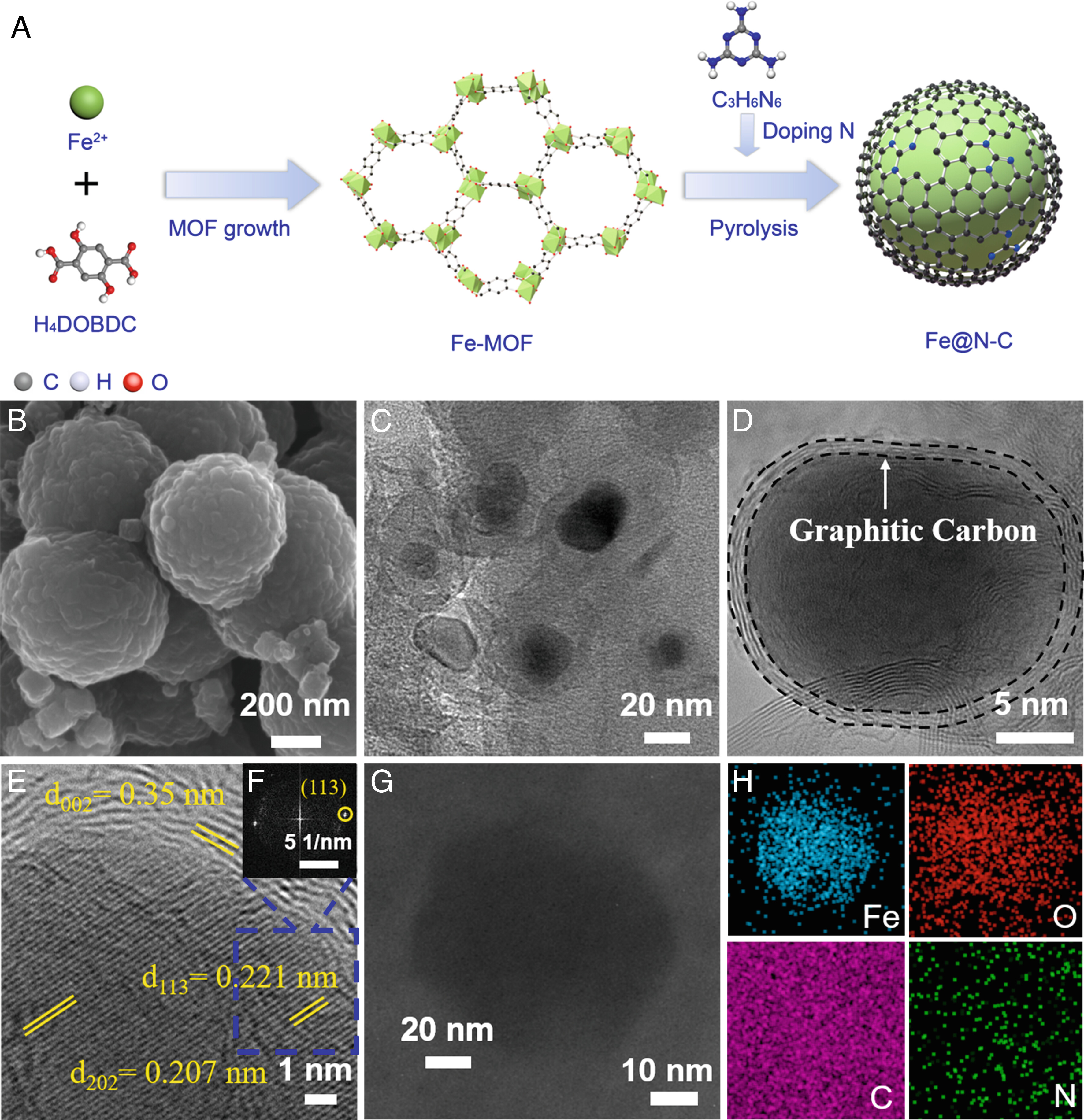
Fig. 1. Synthesis and morphology of Fe@N10-C.
Next, we assessed the structure-property relationship of these catalysts, and extended X-ray absorption fine structure measurements were performed to investigate the valence states and coordination environments of the Fe, N, and C sites in Fe@Nx-C. This provides further evidence of the formation of N-doped C during the pyrolysis process. In addition, after pyrolysis treatment, the N specie in the Fe@Nx-C catalysts was mainly graphitic-N. Therefore, the dominant reactive sites for Fe@Nx-C can be postulated to be C active sites neighboring the N sites (CN). The enhanced activity of the Fe@N10-C catalyst can be attributed to the appropriate amount of N-dopant in the carbon layers surrounding the Fe NPs.
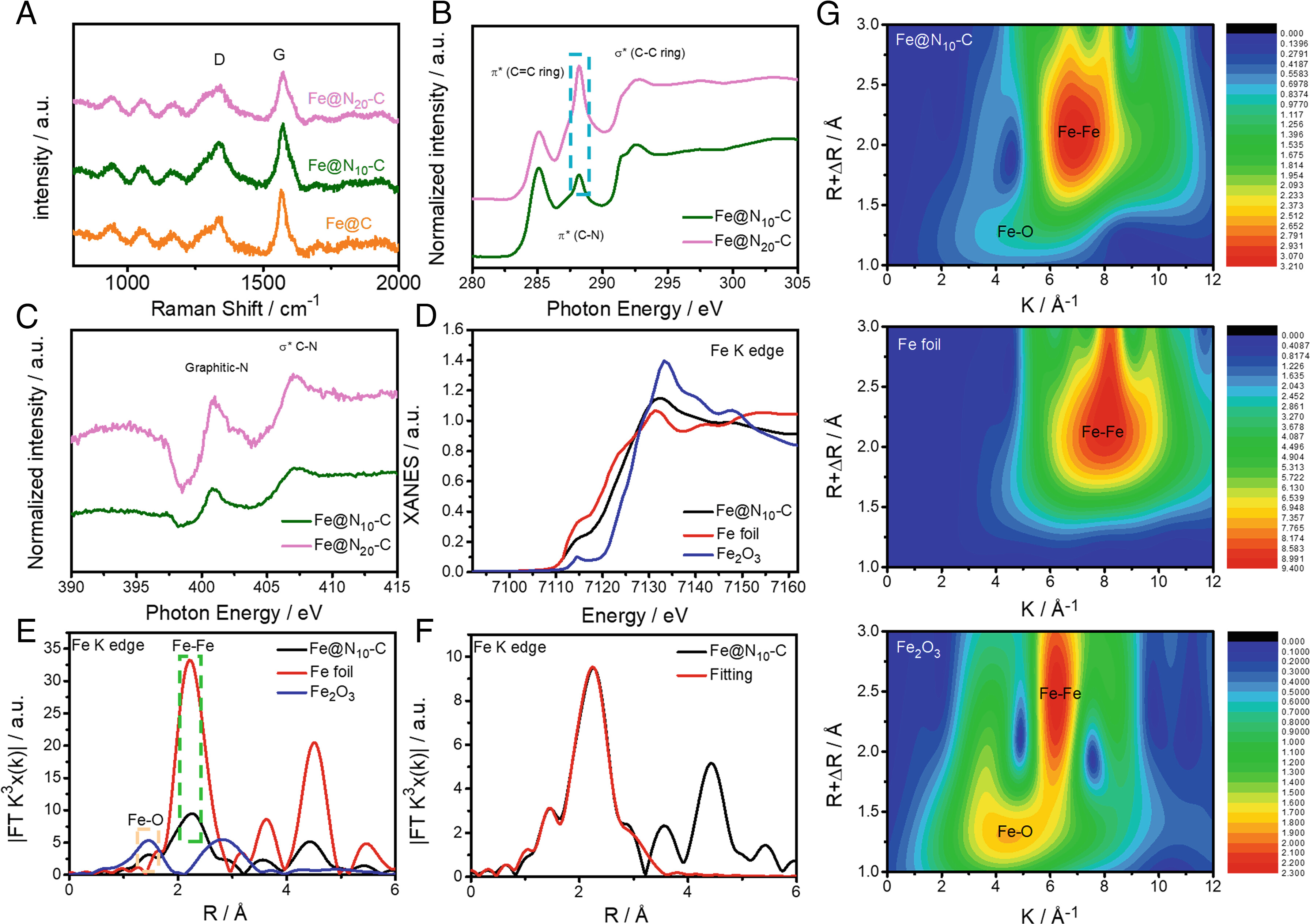
Fig. 2. Characterisation of Fe@N10-C.
To investigate the role of the nitrogen species in activating the C atom and enhancing the NO3-RR activity of Fe@N10-C, density functional theory (DFT) calculations were carried out using the Vienna Ab Initio Simulation Package. To reveal the active sites of the three representative models, Fe@N, Fe@N10-C, and Fe@N20-C, electron density difference maps were calculated. As shown, charge transfer occurs at the C site. Moreover, N doping causes distortions in the neighboring C atoms. Thus, the effect of N doping on the electronic structure was investigated. There is a more pronounced charge accumulation at the CN sites, suggesting that N doping increases the charge density and, thus, facilitates NO3- adsorption. Bader charge analysis was then used to determine the electron loss or gain per C atom. The Bader charge of the C atoms in Fe@Nx-C varied significantly compared to that of Fe@C, indicating a redistribution of electrons. On the basis of the Bader analysis, the CN sites showed greater charge transfer, further indicating that CN is the active site. To better understand the C atom activation and the origin of the activity of the Fe@N10-C catalyst, we investigated the interactions between the Fe and N-doped carbon. The charge density difference plot suggests that N doping affects electrons transferred from the Fe NPs to N-doped carbon results in activated C atom, which further affects the adsorption of reactant molecules and boosts the reaction kinetics.
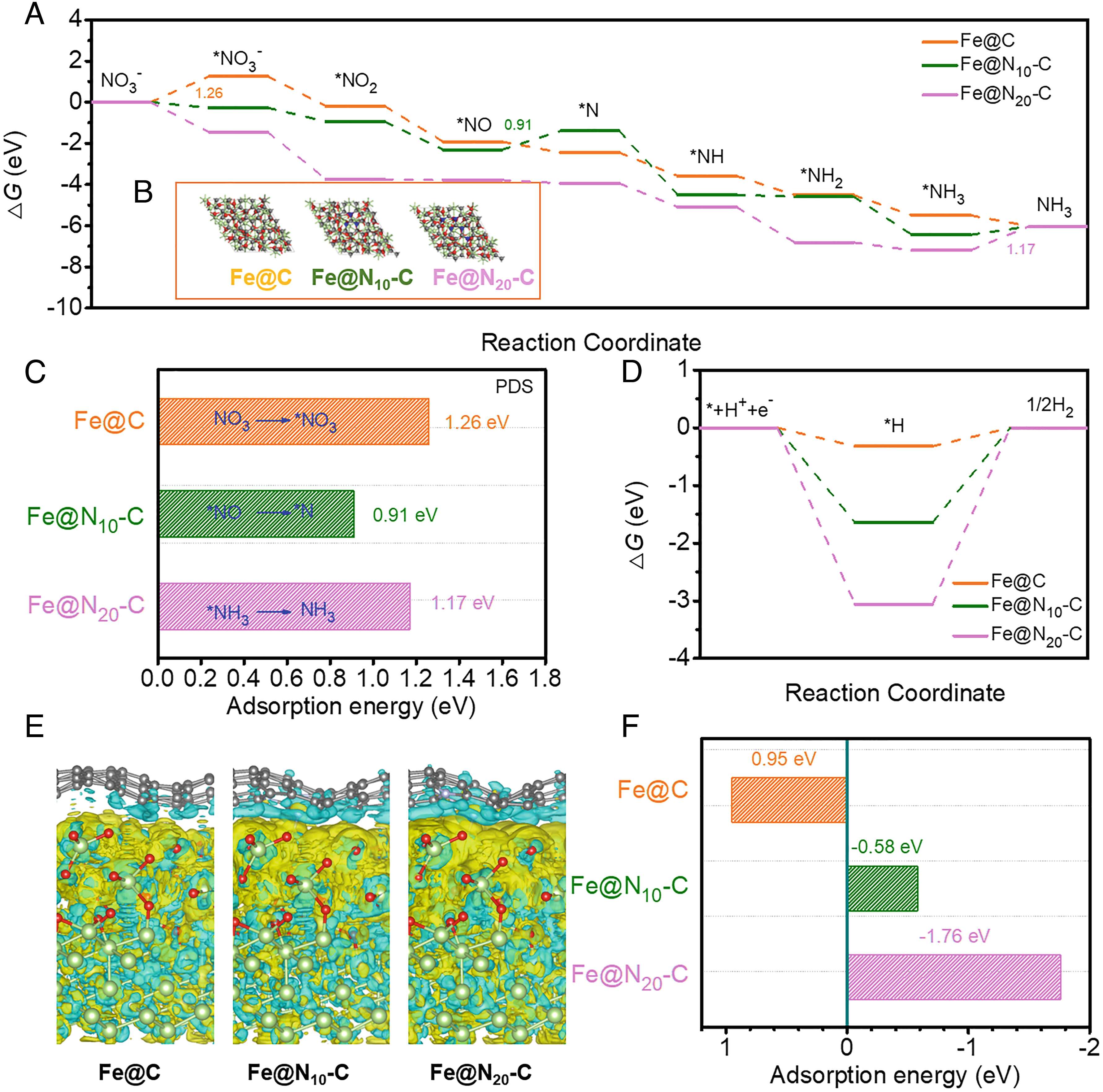
Fig. 3. Mechanistic study of catalytically active sites in Fe@N10-C for NO3-RR.
The first author of the paper is Shuo Zhang, a doctoral candidate of the school of environment in Tsinghua University. The corresponding author of the paper is Miao Li, an associate professor of the school of environment in Tsinghua University. The Professor Xiang Liu have provided important guidance and help for the experimental research and analysis. The research project is supported by project of the National Natural Science Foundation of China.
Paper link: https://doi.org/10.1073/pnas.2207080119