A new cathode design brings energy-dense and environmentally friendly lithium-carbon dioxide batteries closer to reality.
A novel bidirectional catalyst, designed at Tsinghua University, has overcome a key issue hindering the development of rechargeable lithium-carbon dioxide (Li-CO2) batteries. Still in the early stages of development, potential uses for these unusual batteries range from powering rovers under the harsh conditions of Mars, to utilizing waste carbon dioxide here on Earth.
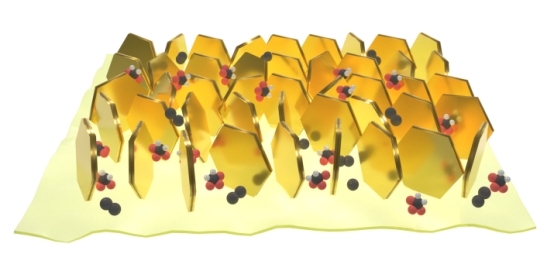
The team’s new catalyst, composed of MoS2 (hexagons) growing vertically on Co9S8 (yellow base), has large number of exposed, active edge sites.
Li-CO2 batteries, by design, use up carbon dioxide. As the batteries discharge, lithium and carbon dioxide are converted into the solids lithium carbonate and carbon, potentially locking away the greenhouse gas.
This also makes them suitable for use in environments with high proportions of carbon dioxide, such as the atmosphere of Mars or on Earth-bound submarines.
These batteries could also be used in conjunction with the carbon capture and storage technologies increasingly being installed at power plants and other industrial sites — where they would turn waste carbon dioxide into green electricity.
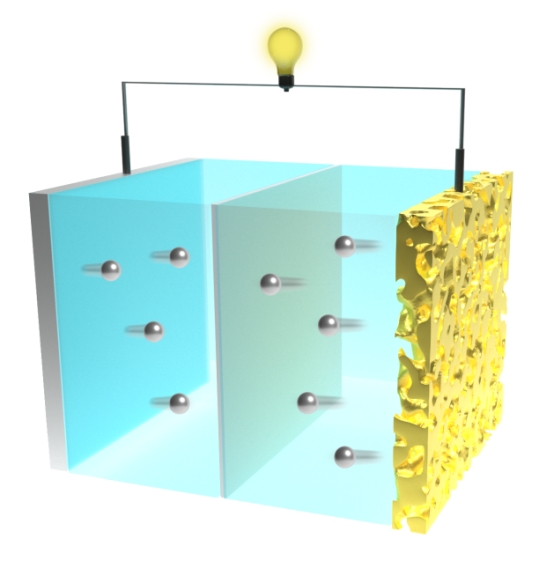
The new two-dimensional, called V-MoS2/Co9S8@CP (in yellow), has been designed for use in rechargeable lithium-carbon dioxide batteries (pictured).
Green power
“The use of Li-CO2 batteries is a win-win situation, providing a new method for CO2 fixation and also opportunities in CO2-rich environments,” says lead researcher, Guangmin Zhou, a chemical engineer at the Tsinghua-Berkeley Shenzhen Institute and Tsinghua Shenzhen International Graduate School.
It is anticipated that Li-CO2 batteries could eventually offer energy densities five to 10 times greater than traditional lithium ion batteries. Researchers hope that Li-CO2 batteries will find uses in the aviation and aerospace industries, which are continually searching for smaller and lighter batteries.
A typical Li-CO2 battery design has a lithium-metal anode, a non-aqueous electrolyte, and a porous cathode. The cathode is the site where carbon dioxide is reduced to form lithium carbonate and carbon during battery discharge, and also the site of the reverse reaction during recharge.

A scanning electron microscope of V-MoS2/Co9S8@CP at a micrometre scale.
But both these reactions are sluggish with current cathode designs, explains Zhou, leading to poor reaction reversibility and low energy efficiency of the battery.
Zhou’s team recently detailed an improved porous cathode design in the journal PNAS (Proceedings of the National Academy of Sciences).
The scientists’ cathode is comprised of ultra-small molybdenum disulfide (MoS2) crystals grown vertically on sheets of cobaltpentlandite (Co9S8), supported by carbon paper.
Impressive efficiency
There are locations, called active sites, on both the MoS2 and Co9S8 where the reactions take place. “We designed a two-dimensional material with a large number of exposed dual active sites — MoS2 edge sites and Co9S8 active sites — which show obviously synergistic effects to facilitate the reaction kinetics,” says Zhou.
As well as providing numerous active sites of both types, the pores in the sheets were designed to facilitate diffusion of carbon dioxide and electrolyte through the materials. The pores also provide ample storage space for the solid discharge products.
The team’s experimental measurements and density functional theory calculations suggest that the new cathode design has an impressive energy efficiency of more than 81%.
However, there is still work to be done before Li-CO2 batteries can reach their full potential, says Zhou. This includes finding suitable electrolytes and perfecting anode design. Stable lithium ‘plating/stripping’ at the anode to prevent unwanted build-ups of the metal is “essential for long-life and safe Li-CO2 batteries,” Zhou adds.

Guangmin Zhou (at center), an associate professor at the Tsinghua-Berkeley Shenzhen Institute and Tsinghua Shenzhen International Graduate School, is creating highly efficient cathode materials for use in rechargeable lithium-carbon dioxide batteries.
Reference
Lu, B. et al. Engineering the interfacial orientation of MoS2/Co9S8 bidirectional catalysts with highly exposed active sites for reversible Li-CO2 batteries. PNAS 120 (6), e2216933120 (2023) DOI: 10.1073/pnas.2216933120
Editor: Guo Lili